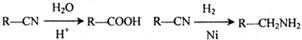��Ŀ����
I���״���һ�ֿ�����ȼ�ϣ����ķе�Ϊ64��7oC���п�ѧ��������Ѻ��й���CO2�Ŀ�������̼�����Һ�У�Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��õ��״����乹���������£�
�Իش��������⣺
��1��д�����ճ�����Ҫ��Ӧ�Ļ�ѧ����ʽ ��
��2����2��105pa��300��ϳ����У�����440gCO2��H2ǡ����ȫ��Ӧ���ɼ״���ˮ���ų�495kJ����������д���ϳ����з�����Ӧ���Ȼ�ѧ����ʽ ��
��3���״���һ����Ҫ�����ǿ�����ȼ�ϵ�أ�����KOH���������Һ�������ĵ缫��ӦʽΪ��
��
�������ȣ�C1O2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
��1����ҵ���Ʊ�C1O2�ķ�Ӧԭ�������ã�2NaC1O3+4HC1=2C1O2��+C12��+2H2O+2NaC1������Ӧ�в���0.1mo1C1O2����ת�Ƶ��ӵ����ʵ���Ϊ________mol��
��2��Ŀǰ�ѿ������õ�ⷨ��ȡC1O2���¹��ա�
����ͼʾ����ʯī���缫����һ�������� ��ⱥ��ʳ��ˮ��ȡC1O2��д����������C1O2�ĵ缫��Ӧʽ��____��
�ڵ��һ��ʱ�䣮�������������������Ϊ112mL����״����ʱ��ֹͣ��⡣
ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ mol;��ƽ���ƶ�ԭ������������pH�����ԭ�� ��
��3��C1O2����ˮ��Fe2+��Mn2+��S2����CN���������Ե�ȥ��Ч����ij������ˮ�к�CN��������C1O2��CN��������������������Ϊ���壬�����ӷ�Ӧ����ʽΪ ��
I��(1)K2CO3+CO2+H2O=2KHCO3 (2)CO2��g��+3H2��g����CH3OH��l��+H2O��l����H=-49.5kJ/mol;
��3��CH3OH+8OH--6e-=CO32-+6H2O
��(1)0.1 (2)��Cl-+2H2O-5e-=ClO2��+4H+ ��0.01 ����������2H2O+2e-=H2��+2OH-��������Ũ�ȼ�С��ʹˮ�ĵ���ƽ�������ƶ�������������Ũ������pHֵ����3��2ClO2+2CN-=N2��+2CO2��+2Cl-
�������������I����1����Ӧ��Ϊ������̼��̼�����Һ�������뵽������̼����أ����ݷ�Ӧ��Ͳ��ﲻ��д����ػ�ѧ����ʽ����2����д����ѧ����ʽ����ע�����ʾۼ���״̬��CO2��g��+3H2��g����CH3OH��l��+H2O��l����10molCO2�ų�����495kJ������1molCO2�ų�����49.5kJ����������д����ػ�ѧ����ʽ��
��3��ȼ�ϵ����ȼ���ڸ���ʧȥ���ӷ���������Ӧ�������������õ����ӷ�����ԭ��Ӧ������������ԭ��Ӧ�����غ�͵���غ㣬���Ե������Һ�е缫��ӦΪ��CH3OH+8OH--6e-=CO32-+6H2O��2CH3OH+16OH--12e-=2CO32-+12H2O��
��1�����������Ļ�ѧ����ʽ�����ǿɵõ�ÿ����2molClO2��ת��2mol���ӣ����Է�Ӧ�в���
0.1mo1C1O2����ת�Ƶ��ӵ����ʵ���Ϊ0.1mol����2���������������������ұߣ��ұ�Ϊ���������Ϊ
��������Ӧ��ΪCl-������ΪClO2������д�������缫����ʽ��Cl-+2H2O-5e-=ClO2+4H+�� �������ܹ�
��õ��ӵı�ȻΪˮ�е������ӣ������缫����ʽ��2H2O+2e-=H2��+2OH-���������������������Ϊ
112mL����״����ʱ����0.005molH2��ת��0.01mol���ӣ���Ȼͨ�������ӽ���Ĥ�������ӵ����ʵ�
��Ϊ0.01mol����3�����ݷ�Ӧ��Ͳ��ﲻ��д����ص����ӷ���ʽ��
���㣺�����Ȼ�ѧ����ʽ��д��ȼ�ϵ�ص缫��Ӧ��д����Ŀ�ۺ���ǿ����ǿ�������Ŀ��顣

����
| A��(CH3)3CCH2OH�� | B��(CH3)3COH�� | C��(CH3)2CHOH�� | D��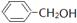 ������� ������� |
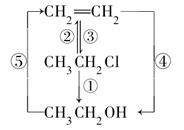
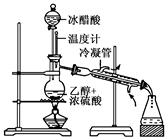
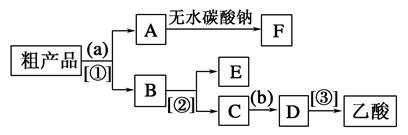
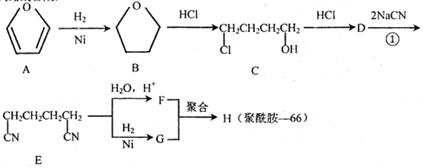
��2������ȥ��ϩ���� ��
��3��D����ᷢ��������Ӧ�IJ����� ��
��ԭ[(C6H10O5)n]��һ����Է��������ȵ��۸���Ķ��ǣ���Ҫ�����ڸ���ͼ����У�������Ϊ������ۣ������й���ԭ��������ȷ���� (����)��
| A����ԭ����ά�ػ�Ϊͬ���칹�壬����ۻ�Ϊͬϵ�� |
| B����ԭˮ������ղ����������� |
| C����ԭ���л�ԭ�ԣ��ǻ�ԭ���� |
| D�������еļ���ԭ�Ǽ����������Ĵ�����ʽ���˶���Ҫ���ܣ��������ֱ�����ü���ԭ |