��Ŀ����
����Ŀ��������W��ҩ��ϳɵ��м��壬����һ�ֺϳ�·�����£�

�ش��������⣺
(1)�Լ�a��___________��
(2)A��������___________��F�й����ŵ�������___________��
(3)����˵����ȷ����___________(����ȷѡ����)��
a��CH2= CHCHO������˳���칹�� b��D�ܷ���ˮ�ⷴӦ
c��E�ķ���ʽΪC9H14NO2 d������˻�ԭ��Ӧ
(4)��Ӧ�۵Ļ�ѧ����ʽ��___________��
(5)G(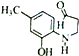 )�ж���ͬ���칹�塣���ڶ������ұ����Ϻ�һNH2��ͬ���칹����___________�֣����к˴Ź���������ʾΪ6��壬�ҷ����֮��Ϊ3��2��2��2��2��2�Ľṹ��ʽΪ___________��
)�ж���ͬ���칹�塣���ڶ������ұ����Ϻ�һNH2��ͬ���칹����___________�֣����к˴Ź���������ʾΪ6��壬�ҷ����֮��Ϊ3��2��2��2��2��2�Ľṹ��ʽΪ___________��
(6)  ����ҩ�м��壬����������̺���ѧ֪ʶ�����DΪ��ʼԭ�Ϻϳ�
����ҩ�м��壬����������̺���ѧ֪ʶ�����DΪ��ʼԭ�Ϻϳ� �ĺϳ�·��ͼ��___________(���Լ���ѡ)��
�ĺϳ�·��ͼ��___________(���Լ���ѡ)��
���𰸡�Ũ���ᡢŨ���� �������ױ� �ǻ������� ab 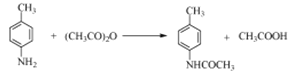 6
6 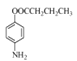

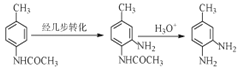
��
��������
�ɺϳ�·�߿�֪���ױ���Ũ���ᡢŨ������ȷ���������Ӧ������õ��������ױ����������ױ�����������������£�������ԭ��Ӧ���ɶԼ��������Լ���������������Ӧ�õ�D��D������ת���õ�E��E����Ӧ��ת��ΪF��F![]() G��F�����е�-NH2��H2C=CH-CHO�����ļӳɷ�Ӧ��G��Q���ȼӳ�����ȥ�����Q��W������ȥ��Ӧ�õ�W���ݴ˷������
G��F�����е�-NH2��H2C=CH-CHO�����ļӳɷ�Ӧ��G��Q���ȼӳ�����ȥ�����Q��W������ȥ��Ӧ�õ�W���ݴ˷������
(1)���ݺϳ�·��������Ϣ����Ӧ��Ϊ�ױ���Ũ���ᡢŨ������ȷ���������Ӧ�������Լ�a��Ũ���ᡢŨ���ᣬ
�ʴ��ǣ�Ũ���ᡢŨ���
(2)��A�Ľṹ��ʽ��֪��A�������Ƕ������ױ���F�й����ŵ��������ǻ���������
�ʴ��ǣ��������ױ����ǻ���������
(3) a��CH2= CHCHO���������е�1��̼ԭ������������ԭ�ӣ����Բ�����˳���칹�壬��a��ȷ��
b��D�Ľṹ��ʽΪ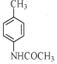 �������к���-NHCO-�������ܷ���ˮ�ⷴӦ����b��ȷ��
�������к���-NHCO-�������ܷ���ˮ�ⷴӦ����b��ȷ��
c��E�Ľṹ��ʽΪ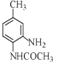 ����ʽΪC9H12NO����c����
����ʽΪC9H12NO����c����
d����Ӧ�����Q��W�����ڵ���ȥ��Ӧ�õ�W��Ҳ����ȥ��������Ӧ����d����
�ʴ��ǣ�ab��
(4)��Ӧ���ǶԼ���������������Ӧ�õ�D����ѧ����ʽ�� ��
��
�ʴ��ǣ� ��
��
(5)G(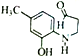 )�����к���ȩ���������ǻ���ͬ���칹�����ڶ������ұ����Ϻ�һNH2��ͬ���칹���У�-NH2��CH3CH2CH2CO-����CH3��2CH CO-�ֱ����ڡ��䡢�Ը�3�֣���6�֣�
)�����к���ȩ���������ǻ���ͬ���칹�����ڶ������ұ����Ϻ�һNH2��ͬ���칹���У�-NH2��CH3CH2CH2CO-����CH3��2CH CO-�ֱ����ڡ��䡢�Ը�3�֣���6�֣�
���к˴Ź���������ʾΪ6��壬�ҷ����֮��Ϊ3��2��2��2��2��2�Ľṹ��ʽΪ ��
��
�ʴ��ǣ�6�� ��
��
(6) �����������̣���Ҫ�� Ϊ��ʼԭ�Ϻϳ�
Ϊ��ʼԭ�Ϻϳ� ����Ҫ�ȷ���������Ӧ����-NO2���ٻ�ԭΪ���������ˮ��õ�����
����Ҫ�ȷ���������Ӧ����-NO2���ٻ�ԭΪ���������ˮ��õ����� ���ϳ�·��ͼ���Ϊ��
���ϳ�·��ͼ���Ϊ�� �������������Ϣ���Ϊ��
�������������Ϣ���Ϊ�� ��
��
�ʴ��ǣ� ��
�� ��
��

 ��У����ϵ�д�
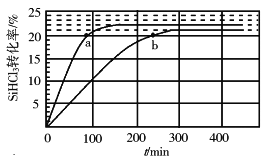
��У����ϵ�д�����Ŀ���о���ѧ��Ӧʱ����Ҫ�������ʱ仯�������仯����Ҫ��ע��Ӧ�Ŀ������ȡ��ش���������:
(1)NH3��ԭNO����Ҫ�����������������䷴Ӧ������������ϵ��ͼ��ʾ

����ͼ����Ϊ�ı��˷�Ӧ��������Ӧ�Ļ�ܣ�b_______(����������������������)a��
��������Ӧ���Ȼ�ѧ����ʽ�ɱ�ʾΪ��Ӧ�����������H��______(��E1��E2�Ĵ���ʽ��ʾ)��
���о����֣�һ�������µ�������Ӧ���̿�����ͼ��ʾ������������ԭ��Ӧ�����ʵ����ã�NOΪ_______���������ܷ�Ӧ�Ļ�ѧ����ʽΪ_______________��

(2)һ���¶��£�����ͬ���ʵ�����H2O(g)��CO�ֱ�ͨ���ݻ�Ϊ1L�ĺ����������У����з�ӦH2O(g)��CO(g)![]() CO2(g)��H2(g)���õ������ʾ����������
CO2(g)��H2(g)���õ������ʾ����������
������ | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ��ʱ��/min | ||
H2O(g) | CO(g) | CO(g) | H2(g) | |||
1 | 650 | 2.0 | 4.0 | 3.0 | 1.0 | 5 |
2 | 900 | 1.0 | 2.0 | 1.8 | 0.2 | 4 |
3 | 900 | a | b | c | d | t |
��4mim�ڣ�ʵ��2��v(CO2)��______�� 900��ʱ����Ӧ��ƽ�ⳣ��Ϊ______�������¶�ʱ��ƽ�ⳣ����________(��������������С������������)��
��650��ʱ�����ڴ������г���2.0 mol H2O(g)��1.0molCO(g)��1.0 mol CO2(g)�� xmol H2(g)��Ҫʹ��Ӧ�ڿ�ʼʱ������Ӧ������У���xӦ�����������__________��
��a��2.0��b��1.0����ƽ��ʱʵ��2��H2O(g)��ʵ��3��CO(g)��ת����(a)�Ĺ�ϵΪa(H2O) _______ (�������������ɣ���)a(CO)��