��Ŀ����
13�����������ƣ�Na2S2O5���dz��õ�ʳƷ��������֮һ���ױ�����Ϊ�����ƣ�ij�о�С���������ʵ�飺������ͼװ�ã�ʵ��ǰ�ѳ���װ���ڵĿ�������ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪ��Na2SO3+SO2�TNa2S2O5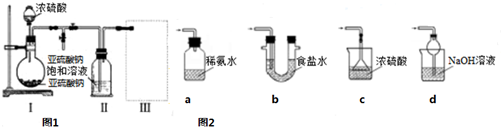
��1��װ�â��в�������Ļ�ѧ����ʽΪNa2SO3+H2SO4=Na2SO4+SO2��+H2O��
��2��Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽���ǹ��ˣ�
��3��װ�â����ڴ���β������ѡ����ͼ2�������װ�ã��г���������ȥ��Ϊd������ţ���
��4��Na2S2O5����ˮ������NaHSO3��֤��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õķ�����ae������ţ���
a���ⶨ��Һ��pH b������Ba��OH��2��Һ c���������� d������Ʒ����Һ e������ɫʯ����ֽ���
��5������Na2S2O5�����ڿ������ѱ�������ʵ�鷽����ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ�
��6�����ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�������������SO2���㣩�ķ�����ͼ��

����֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2+I2+2H2O�TH2SO4+2HI��
�ٰ���������ʵ�飬���ı�I2��Һ25.00mL���ô�ʵ������Ʒ�п��������IJ�������������SO2���㣩Ϊ0.16g•L-1��
��������ʵ������У����в���HI���������������ý��ƫ�ͣ��ƫ�ߡ���ƫ�͡����䡱����
���� ��1����װ�â��з����ķ�Ӧ��֪��װ�â��в���������ΪSO2���������������ᷴӦ���������ơ�����������ˮ��
��2��װ�â��л���������ľ��壬���������Һ̬��Ӧ��ȡ���˲�����
��3��װ�â����ڴ���β��������Ϊ��Ӧ�Ķ�������Ӧ��ֹ�������Ҳ��ܴ�����ȫ�ܱջ����У�
��4��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ��ʼ�����Һ�����Լ��ɣ�
��5��Na2S2O5�����ڿ������ױ�����ΪNa2SO4�������ᡢ�Ȼ�����Һ������Ʒ���Ƿ�����������ɣ�
��6���������ĵ���������SO2+I2+2H2O�TH2SO4+2HI��������������������������Ũ�ȣ�
�����в���HI�����������������ĵ����ƫС���ʲⶨ�����������ƫС��
��� �⣺��1����װ�â��з����ķ�Ӧ��֪��װ�â��в���������ΪSO2���������������ᷴӦ���������ơ�����������ˮ����Ӧ����ʽΪNa2SO3+H2SO4=Na2SO4+SO2��+H2O��
�ʴ�Ϊ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
��2��װ�â��л���������ľ��壬���������Һ̬��Ӧ��ȡ���˽��з��룬�ʴ�Ϊ�����ˣ�
��3��a��װ��Ӧ���������백ˮ�п������ն�������Ϊ�ܱջ�����װ����ѹǿ�����ײ�����ȫ�¹ʣ��ʴ���
b����װ�����ն������������ϲ��Ϊ�ܱջ�����װ����ѹǿ�����ײ�����ȫ�¹ʣ��ʴ���
c����װ�ò������ն�������������ʵ��ʵ��Ŀ�ģ��ʴ���
d����װ���������������������Ӧ���������գ��ҷ�ֹ����������ȷ��
�ʴ�Ϊ��d��
��4��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ��ⶨ��Һ��pH������ȷ����Һ����ԣ�������Һ����ʹʪ����ɫʯ����ֽ��죬�������òⶨ��ҺpHֵ��ʪ�����ɫʯ����Һ���飬������Ba��OH��2��Һ��HCl��Һ��Ʒ����Һ������˵����Һ�����ԣ���ѡae��
�ʴ�Ϊ��ae��
��5��Na2S2O5��SԪ�صĻ��ϼ�Ϊ+4�ۣ���˻ᱻ����ΪΪ+6�ۣ��������ڿ������ױ�����ΪNa2SO4�������ᡢ�Ȼ�����Һ������Ʒ���Ƿ�����������ɣ�ʵ�鷽��Ϊ��ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ�
�ʴ�Ϊ��ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ�
��6������100mL���Ѿ��ж������������Ϊmg����
SO2+2H2O+I2�TH2SO4+2HI
64g 1mol
mg 0.025L��0.01mol/L
���ԣ�64g��mg=1mol��0.025L��0.01mol/L��
���m=0.016
�ʸô�ʵ������Ʒ�п��������IJ�������������SO2���㣩Ϊ$\frac{0.016g}{0.1L}$=0.16 g/L
�ʴ�Ϊ��0.16��
�����в���HI�����������������ĵ����ƫС���ʲⶨ�����������ƫС����ⶨ���ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
���� ���⿼�����ʵ��Ʊ�ʵ�顢ʵ�鷽����ơ����ʺ����IJⶨ��������ԭ��Ӧ�ζ��ȣ��Ѷ��еȣ���ȷʵ��ԭ���ǽⱾ��ؼ����������ʵ����ʷ������ע��Ԫ�ػ�����֪ʶ�Ļ��ۺ�������ã�

�ٻ���������Һ�����ᡢˮ��
�ڻ�����Ȼ��ء����顢HD
�۵���ʣ����������������ᱵ
�ܷǵ���ʣ��ƾ���CO2��Cl2
��ͬλ�أ�12C��13C��14C��
| A�� | �ۢ� | B�� | �ۢ� | C�� | �ڢۢ� | D�� | �ڢ� |
��1���Ƚ�a��b���������=����������
��2���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1��T2���������=����������
| T/K | T1 | T2 | T3 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
��3������ͬ������Ҫ��õ�2a kJ��������������ʵ����ʵ���������D
A.4mol A��2mol B B.4mol A��2mol B��2mol C
C.4mol A��4mol B D.6mol A��4mol B
��4��������������Ϊ��ѹ��������Ӧǰ�����ͬ������ʼʱ����2mol A��1mol B��500��ʱ��ַ�Ӧ��ƽ��ų�����Ϊd kJ����d��b���������=��������
��5��һ���¶��£���һ���ݻ��ɱ�������У�ͨ��3mol A��2mol B�����������ʹ��Ӧ��ƽ��ʱ�������������ʵ���Ϊ��ʼʱ��90%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼ���ʵ�����Ϊ4mol A��3mol B��2mol C����ƽ��ʱA�İٷֺ������� ������䡱�������С������ȷ��������
| ѡ�� | �� | �� | �� | ʵ����� | 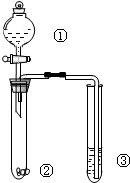 |
| A | Ũ���� | KMnO4 | NaBr��Һ | �����ԣ�KMnO4��Cl2��Br2 | |
| B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
| C | ϡ���� | Na2SO3 | Ba��NO3��2��Һ | SO2������Ա��ξ������ɰ�ɫ���� | |
| D | ϡ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ����̼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 1��1 | B�� | 1��3 | C�� | 3��2 | D�� | 2��3 |
�ٲ�����ˮ���Σ�CaCO3��BaSO4�ȣ������������
��pH=1��ǿ����Һ����ˮϡ�ͺ���Һ����������Ũ�ȶ�����
�۷�Ӧ4A ��g��+B��g���T2C��g��+D��g�������Է����У���÷�Ӧ��Hһ��С��0
����Һ�����塢��Һ���ַ�ɢϵ���ö����ЧӦ��������
�����ڵĵ���ʶ��ܵ���
����������ԭ�������жϿ��淴Ӧ�Ƿ�ﵽƽ��״̬��
| A�� | �٢ۢݢ� | B�� | �ڢܢݢ� | C�� | ֻ�Т� | D�� | ������ |
| A�� | 0.3 mol•L-1 | B�� | 0.4 mol•L-1 | C�� | 0.5 mol•L-1 | D�� | 0.6 mol•L-1 |

| A�� | �������� | B�� | �ҿ��������� | ||
| C�� | �������������� | D�� | ���������������� |