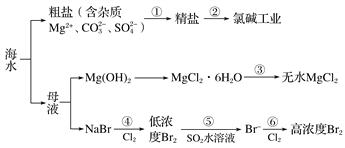
��Ŀ����
����Ŀ����ͼ��ʾ����һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʺͷ�Ӧ��������ȥ��������A�Ǽ�ͥ�����г��õ�һ�ֵ�ζƷ����Ӧ�ٳ�֮Ϊ���ȼҵ����D�ǻ���ɫ���壬H��ˮ��Һ����Ư�ס�ɱ���ԣ�JΪ���ɫ������
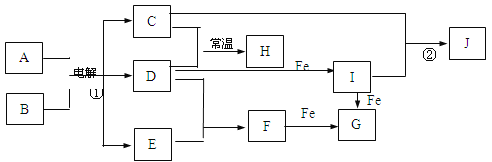
��1��H�Ļ�ѧʽΪ____________��
��2��д��I��Fe��Ӧ����G�����ӷ���ʽΪ_____________________________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ____________________________________________��
��4����Ӧ�ڵ����ӷ���ʽΪ____________________________________________��
���𰸡�NaClO 2Fe3++ Fe=3Fe2+ 2NaCl+2H2O![]() 2NaOH+Cl2��+H2�� Fe3++3OH-= Fe(OH)3��
2NaOH+Cl2��+H2�� Fe3++3OH-= Fe(OH)3��
��������
��1���������֪����Ϊ��ⱥ��ʳ��ˮ��ʵ�飬AΪNaCl��BΪH2O��CΪNaOH��DΪCl2,��HΪNaClO��
��2��IΪFeCl3�����Fe��Ӧ�����ӷ���ʽΪ2Fe3++ Fe=3Fe2+
��3����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ2NaCl+2H2O=2NaOH+Cl2��+H2��
��4����Ӧ����FeCl3��NaOH�ķ�Ӧ�������ӷ���ʽΪFe3++3OH-= Fe(OH)3��

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ