��Ŀ����
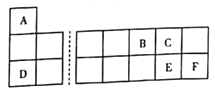
����Ŀ��A��B��C��D��E��F���ֶ�����Ԫ����Ԫ�����ڱ��е�λ����ͼ��ʾ��
�ش��������⣺
��1�������ڱ���,Eλ�ڵ�_____________���ڵ�_____________�塣
��2��A��B����ɼȺ����Լ��ֺ��Ǽ��Լ��Ļ���������ʽΪ_____________��
��3����E��F����̬�⻯����,���ȶ��Խ�ǿ����_____________(�ѧʽ,��ͬ),��ԭ�Խ�ǿ����_______________��
��4��A��C��D��E���������ʽ��X��Y,��X��Y��Һ��Ϸ������ֽⷴӦ,д�����ӷ���ʽ��________________________________________________________��
��5������(BA4)2E2C8�����ˮ�е�Mn2+,���۲쵽��ɫ��Һ���Ϻ�ɫ,���жϷ�ˮ�к�Mn2+,��ԭ�����������ữ��BaCl2��Һ��ϲ�����ɫ������д����ɫ��Һ���Ϻ�ɫ��Һ�����ӷ���ʽ��_________________________________________________��
���𰸡� �� ��A 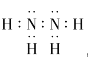 HCl H2S HSO3-+H+=SO2��+H2O 5S2O82-+2Mn2++8H2O=10SO42-+2MnO4-+16H+
HCl H2S HSO3-+H+=SO2��+H2O 5S2O82-+2Mn2++8H2O=10SO42-+2MnO4-+16H+
�������������������ͼ��֪��A��B��C��D��E��F�ֱ�ΪH��N��O��Na��S��Cl�����ֶ�����Ԫ����
��1�������ڱ���,Eλ�ڵ������ڵڢ�A�塣
��2��A��B����ɼȺ����Լ��ֺ��Ǽ��Լ��Ļ�����ΪN2H4�������ʽΪ ��
��
��3��S��Cl�ķǽ����������S<Cl����������̬�⻯�������ȶ��Խ�ǿ����HCl����ԭ�Խ�ǿ����H2S��
��4��A��C��D��E���������ʽ��Ϊ�������ƺ����������ƣ�������Һ��Ϸ������ֽⷴӦ�������ӷ���ʽΪHSO3-+H+=SO2��+H2O��
��5������(NH4)2S2O8�����ˮ�е�Mn2+,���۲쵽��ɫ��Һ���Ϻ�ɫ,���жϷ�ˮ�к�Mn2+,��ԭ�����������ữ��BaCl2��Һ��ϲ�����ɫ��������S2O82-����ԭΪSO42-��Mn2+������ΪMnO4-���÷�Ӧ�����ӷ���ʽΪ5S2O82-+2Mn2++8H2O=10SO42-+2MnO4-+16H+��

 ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�