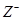��Ŀ����
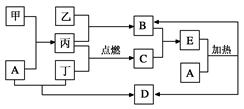
�ɶ����ڳ���Ԫ���γɵĴ�����A��B��C��D��E��F��Xת����ϵ����ͼ��ʾ��ij��������ȥ����

��֪��B��XΪ���ʣ�������DΪ��ɫҺ�壬A��B��ͬһ��Ԫ�ء�
��ش��������⣺
��1����E�����Ǵ�����Ⱦ�F��һԪǿ�ᡣ
��д��E��F��Ӧ�Ļ�ѧ����ʽ�� ��
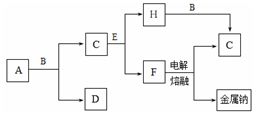
������25��ʱ0.1 mol��L��1 A��ˮ��Һ���������м�������0.1 mol��L��1��ϡ���ᣬ��������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳���� ��
���ڳ����£���V1 L pH��a��A��ˮ��Һ�м���V2 L pH��b�����ᣬ��a��b��14����ǡ����ȫ��Ӧ����V1��V2�Ĺ�ϵΪVl V2���������������������������ȷ����������pH��������Һ������Ƚϣ���ˮ�������c(H��)ǰ��Ϊ���ߵ�108������������Һ��pH�� ��
��2����E���岻�Ǵ�����Ⱦ�F�Ƕ�Ԫ���ᡣ
��B����Ԫ�������ڱ��е�λ�� ��
��д����������C��Ӧ�Ļ�ѧ����ʽ�� ������������Eͨ������������Һ�еò�����F��F��Ksp��2.8��10��9���ֽ��ó�������0.1 mol��L��1��CaCl2��Һ�У���Ksp �����������С�����䡱������ʱ����ɲ�����F������������Һ�е�Ũ��Ϊ ��

��֪��B��XΪ���ʣ�������DΪ��ɫҺ�壬A��B��ͬһ��Ԫ�ء�
��ش��������⣺
��1����E�����Ǵ�����Ⱦ�F��һԪǿ�ᡣ
��д��E��F��Ӧ�Ļ�ѧ����ʽ�� ��
������25��ʱ0.1 mol��L��1 A��ˮ��Һ���������м�������0.1 mol��L��1��ϡ���ᣬ��������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳���� ��
���ڳ����£���V1 L pH��a��A��ˮ��Һ�м���V2 L pH��b�����ᣬ��a��b��14����ǡ����ȫ��Ӧ����V1��V2�Ĺ�ϵΪVl V2���������������������������ȷ����������pH��������Һ������Ƚϣ���ˮ�������c(H��)ǰ��Ϊ���ߵ�108������������Һ��pH�� ��
��2����E���岻�Ǵ�����Ⱦ�F�Ƕ�Ԫ���ᡣ
��B����Ԫ�������ڱ��е�λ�� ��
��д����������C��Ӧ�Ļ�ѧ����ʽ�� ������������Eͨ������������Һ�еò�����F��F��Ksp��2.8��10��9���ֽ��ó�������0.1 mol��L��1��CaCl2��Һ�У���Ksp �����������С�����䡱������ʱ����ɲ�����F������������Һ�е�Ũ��Ϊ ��
��1���� 3NO2+H2O=2HNO3+NO ��c(H+)> c( SO42-) > c(NH4+) >c(OH��) �� �� 3
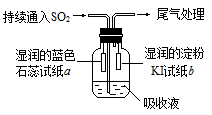
��2���ٵڶ����ڢ�A�� �� Fe2O3+3CO 2 Fe +3CO2 ���� 2.8��10-8 mol��L-1
2 Fe +3CO2 ���� 2.8��10-8 mol��L-1
��2���ٵڶ����ڢ�A�� �� Fe2O3+3CO
 2 Fe +3CO2 ���� 2.8��10-8 mol��L-1
2 Fe +3CO2 ���� 2.8��10-8 mol��L-1���������(1)�������ʼ���ת����ϵ����֪��������֪A��NH3��B��N2��C��NO��D��H2O��E��NO2��F��HNO3��X��O2����д��E��F��Ӧ�Ļ�ѧ����ʽ��3NO2+H2O=2HNO3+NO���ڰ�ˮ������������Ũ�Ȼ�ϵõ���ΪNH4HSO4��Һ��NH4HSO4= NH4++H++SO42-�����ڷ�����NH4++H2O
 NH3��H2O+H+��H2O
NH3��H2O+H+��H2O OH-+H+������������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����c(H+)> c( SO42-) > c(NH4+) >c(OH��)�����ڳ����£�����NH3��H2O pH��a,C(H+)= 10-amol/L ,C(OH-)= 10(a-14)mol/L pH��b�����ᣬC(H+)=10-bmol/L ��a��b��14������C(H+)= C(OH-)�����ڼ�Ϊ����C(NH3��H2O)>C(HCl).��ǡ����ȫ��Ӧ�� V1��V2�Ĺ�ϵ��V1��V2����NH4Cl��HCl��PH��ȣ��������ǵ�PHΪX����ǰ��ˮ���������C(H+)=10-amol/L,������ˮ�������c(H��)Ϊ10-(a+8)mol/L, C(OH-)= c(H��)=10-(a+8)mol/L,��������Һ��C(OH-)��c(H��)=Kw=10-14. 10-(a+8) ��10-a=10-14.���a="3." ��������Һ��pH��3.(2) �������ʼ���ת����ϵ����֪��������֪A��CH4��B��C��C��CO��D��H2O��E��CO2��F��H2CO3��X��O2����B����Ԫ�������ڱ��е�λ�õڶ����ڢ�A�壬��д����������CO��Ӧ�Ļ�ѧ����ʽ��Fe2O3+3CO
OH-+H+������������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����c(H+)> c( SO42-) > c(NH4+) >c(OH��)�����ڳ����£�����NH3��H2O pH��a,C(H+)= 10-amol/L ,C(OH-)= 10(a-14)mol/L pH��b�����ᣬC(H+)=10-bmol/L ��a��b��14������C(H+)= C(OH-)�����ڼ�Ϊ����C(NH3��H2O)>C(HCl).��ǡ����ȫ��Ӧ�� V1��V2�Ĺ�ϵ��V1��V2����NH4Cl��HCl��PH��ȣ��������ǵ�PHΪX����ǰ��ˮ���������C(H+)=10-amol/L,������ˮ�������c(H��)Ϊ10-(a+8)mol/L, C(OH-)= c(H��)=10-(a+8)mol/L,��������Һ��C(OH-)��c(H��)=Kw=10-14. 10-(a+8) ��10-a=10-14.���a="3." ��������Һ��pH��3.(2) �������ʼ���ת����ϵ����֪��������֪A��CH4��B��C��C��CO��D��H2O��E��CO2��F��H2CO3��X��O2����B����Ԫ�������ڱ��е�λ�õڶ����ڢ�A�壬��д����������CO��Ӧ�Ļ�ѧ����ʽ��Fe2O3+3CO 2 Fe +3CO2����Ϊ�������ܶȻ�����KSpֻ���¶��йأ������ӵ�Ũ�ȴ�С�أ����Խ��ó�������0.1 mol��L��1��CaCl2��Һ�У���Ksp���䡣��ʱ����ɲ�����F������������Һ�е�Ũ��Ϊ2.8��10��9��0.1=2.8��10-8 mol/L.
2 Fe +3CO2����Ϊ�������ܶȻ�����KSpֻ���¶��йأ������ӵ�Ũ�ȴ�С�أ����Խ��ó�������0.1 mol��L��1��CaCl2��Һ�У���Ksp���䡣��ʱ����ɲ�����F������������Һ�е�Ũ��Ϊ2.8��10��9��0.1=2.8��10-8 mol/L.
��ϰ��ϵ�д�
�����Ŀ





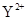 ��Z
��Z ��
��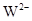 �������Ӿ�������ͬ�ĵ��Ӳ�ṹ�����й���X��Y��Z��W����Ԫ�ص���������ȷ���ǣ� ��
�������Ӿ�������ͬ�ĵ��Ӳ�ṹ�����й���X��Y��Z��W����Ԫ�ص���������ȷ���ǣ� �� ��
��