��Ŀ����
����Ŀ��I.Ԫ�����ڱ���һ�������±���ʾ������Ԫ�آ١����ڱ��е�λ�ã���ش���������:

(1)��������ӽṹʾ��ͼΪ_____________________���۵ļ��⻯����������������Ӧ��ˮ���ﻯ�����ɵ����εĻ�ѧʽΪ______________________��
(2)�ݺ͢��γɵĻ�����ĵ���ʽΪ________________________��
(3)�ۡ��������������Ӧˮ�����������ǿ������˳��Ϊ___________(�û�ѧʽ��ʾ����ͬ)��_________________________��
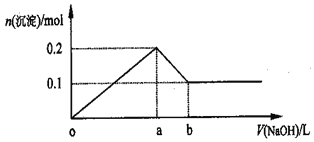
II.ijͬѧ��������ͼװ����֤ͬ���ڻ�ͬ����Ԫ�����ʵݱ���ɡ�

(4)�����D��������_________________________________________��
(5)֤���ǽ�����:Cl��I����A�м�Ũ���ᣬB�м�KMnO4(KMnO4��Ũ���᳣���·�Ӧ��������)��C�мӵ��۵⻯�ػ����Һ���۲쵽C����Һ��������C�Թ��з�����Ӧ�����ӷ���ʽΪ_________________________________________���ӻ��������Ĺ۵㿼�ǣ���װ�õ�ȱ����_____________________________________________________��
(6)֤���ǽ�����:N��C����A�м�ϡ���ᣬB�м�̼��ƣ�C�мӳ���ʯ��ˮ���۲쵽C����Һ����ǵ�����ʵ����Ƶ�ԭ��������____________���Ƚ�Ԫ�طǽ����Ե�ǿ����
���𰸡� ![]() (NH4)2SO4
(NH4)2SO4 ![]() HNO3 H2SiO3 ������ Cl2+2I-=I2+2Cl- û��β������װ�ã��������ɣ� ����������Ӧˮ���������ǿ��
HNO3 H2SiO3 ������ Cl2+2I-=I2+2Cl- û��β������װ�ã��������ɣ� ����������Ӧˮ���������ǿ��
��������������I������Ԫ�����ڱ���֪��ΪH����ΪBe����ΪN����ΪO����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl������Ԫ�������ɡ�������ʵ������Լ�����е���������жϡ�
II����4���л������õ�װ���ܷ�ֹ��������5��������������ǿ�����ԣ��������⻯���Լ������ж���Ҫβ��������������6�����ݷǽ�����Խǿ����ۺ����������Խǿ������
��⣺I������Ԫ�������ڱ��е����λ�ÿ�֪��ΪH����ΪBe����ΪN����ΪO����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl����
��1����������Ӽ�ΪS2-��������18�����ӣ�������16�����ӣ��ʽṹʾ��ͼΪ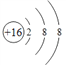 ���۵ļ��⻯��ΪNH3����������������ˮ���TH2SO4��Ӧ�ɵ�����Ϊ����泥���ѧʽΪ(NH4)2SO4��
���۵ļ��⻯��ΪNH3����������������ˮ���TH2SO4��Ӧ�ɵ�����Ϊ����泥���ѧʽΪ(NH4)2SO4��
��2���ݺ͢��γɵĻ�����ΪNaF�������ӻ�����������Ӻͷ����ӹ��ɣ��ʵ���ʽΪ![]() ��
��
��3��Ԫ�صķǽ�����Խǿ������ۺ����������Խǿ�������ڷǽ�����N��Si���ʢۡ��������������Ӧˮ�����������ǿ����ΪHNO3��H2SiO3��
II����4�����θ����D���β��־��нϴ�ռ䣬�������ã��ܹ���ֹ���������Ա���C��Һ�������ƿ�У�
��5��KMnO4��Ũ���ᷴӦ����������2KMnO4+16HCl��2KCl+2MnCl2+5Cl2��+8H2O����������۵⻯�ػ����Һ��Ӧ���ɵⵥ�ʣ���Ӧ���ӷ���ʽΪ��Cl2+2I-��I2+2Cl-������������Һ����ɫ����C����Һ��Ϊ��ɫ����������������ɢ�������У���Ⱦ����������NaOH��Һ���շ�ֹ��Ⱦ��������˴ӻ��������Ĺ۵㿼�ǣ���װ�õ�ȱ����ȱ��β������װ�ã�
��6����A�м�ϡ���ᣬB�м�̼��ƣ����߷�Ӧ��������ơ�������̼��ˮ��ͨ��C�г���ʯ��ˮ����ǿ���֤��CO2�����ɣ���֤������HNO3ǿ��H2CO3������ۺ����������Խǿ����Ԫ�صķǽ�����Խǿ���ʿ���֤���ǽ�����N��C������ʵ����Ƶ�ԭ����������ۺ����������ǿ���Ƚ����Ƚ�Ԫ�طǽ����Ե�ǿ����

����Ŀ��������ͼͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL 0.25mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL 0.55mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ������¶ȡ��ش��������⣺
(1)����NaOH��Һ����ȷ������________��
A���ز����������� B���������������� C��һ��Ѹ�ٵ���
(2)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������________��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β���������ؽ���
(3)ʵ���������±���������д�±��еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ(t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ________ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ��Ƶ���Ϊ0.55mol/L NaOH��Һ��0.25mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к��Ȧ�H��____________________(ȡС�����һλ)��
���к��Ȳⶨʵ���У����в���һ���ή��ʵ��ȷ�Ե���________��
A���õζ���(��������������������0.01)ȡ���������Һ�����
B��NaOH��Һ�ڵ���С�ձ�ʱ������������
C����С�ձ�������ϴв��ŵ�����ĭ���Ͻ϶�
D������HCl��Һ���¶ȼ���ˮϴ�����������NaOH��Һ���¶�