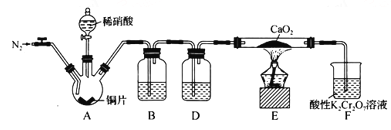
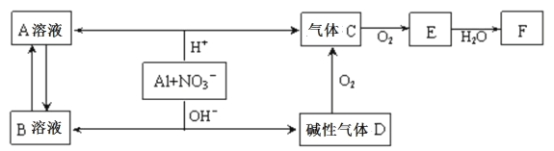
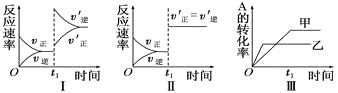
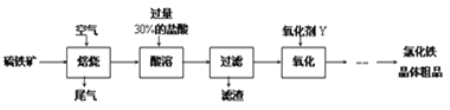
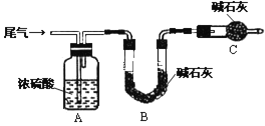
��Ŀ����
����Ŀ����֪A��B��C��D��E��F��G�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ������������������Aԭ�ӵ�L����2��δ�ɶԵ��ӡ�D�ǵ縺������Ԫ�أ�E��Fͬ���壬E�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��G3+����3d�������Ϊ����״̬�����������������ش���������:������ʱ��������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С��������Ϊ______________________��D�ĺ�����______________���˶�״̬��ͬ�ĵ��ӡ�
��2��A������⻯������___________(�������Է����������Ǽ��Է�����)��![]() ���ӿռ乹����__________��������ԭ�Ӳ�ȡ__________________�ӻ���
���ӿռ乹����__________��������ԭ�Ӳ�ȡ__________________�ӻ���
��3�������۵㣺EC_________FC(����>������<������=��)
��4��G��M(������Ϊ25)��Ԫ�صIJ��ֵ������������ڱ���
Ԫ�� | M | G | |
������(kJmol-1) | I1 | 717 | 759 |
I2 | 1509 | 1561 | |
I3 | 3248 | 2957 | |
�Ƚ���Ԫ�ص�I2��I3��֪����̬M2+��ʧȥһ�����ӱ���̬G2+��ʧȥһ�������ѡ���ԭ����_________________________________________��
���𰸡�C<O<N 9 �Ǽ��Է��� ƽ�������� sp2�ӻ� > Mn2+��3d��������Ų�Ϊ����״̬���ȶ�
��������
����ԭ�ӵĽṹ�ص��Ƶ���Ԫ�ط��š�D�ǵ縺������Ԫ�أ���DΪFԪ�أ�Aԭ�ӵ�L����2��δ�ɶԵ��ӣ���AΪC��O����AΪOԪ�أ���B��C�����ܴ��ڣ���AΪCԪ�أ�BΪNԪ�أ�CΪOԪ�أ�E�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����EΪMgԪ�أ�FΪCaԪ�أ�G3+����3d�������Ϊ����״̬��G�ĵ����Ų�ʽΪ1s22s22p63s23d64s2����GΪFeԪ�ء����ϣ�A��B��C��D��E��F��G�ֱ�Ϊ��C��N��O��F��Mg��Ca��Fe��
��1��ͬһ�����ڴ����ң���һ������������Nԭ�ӵ�2p3Ϊ������ṹ�����ȶ��������һ�����ܴ���O�ĵ�һ�����ܣ���C��N��O�ĵ����ܴ�С��ϵΪC<O<N��F������9�����ӣ�ÿ�����ӵ��˶�״̬����ͬ����F��9���˶�״̬��ͬ�ĵ��ӣ�
��2��CH4����ṹΪ�������壬��Ϊ�Ǽ��Է��ӡ�CH3+�ļ۲���Ӷ���Ϊ3��������Ϊ3���µ��Ӷ���Ϊ0����CH3+�Ŀռ乹��Ϊƽ�������Ρ�CH3+�ļ۲���Ӷ���Ϊ3����������ԭ��Cԭ�Ӳ�ȡsp2�ӻ���
��3��MgO��CaO��Ϊ���ӻ����Mg2+��Ca2+���������ͬ������ǰ���뾶�Ⱥ���С������Ϊ�����ܴ��۵������MgO���۵����CaO���۵㡣
��4��MΪMnԪ�أ�Mn2+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d5��Fe2+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d6��Mn2+��3d��������Ų�Ϊ�����״̬�����ȶ��������ʧȥһ�����ӱ�Fe2+���ѡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����¯���������з�����Ӧ��![]() Fe2O3��s��+CO��g��
Fe2O3��s��+CO��g��![]()
![]() Fe��s��+CO2��g������֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����������˵����ȷ���ǣ�������
Fe��s��+CO2��g������֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����������˵����ȷ���ǣ�������
�¶� | 1000 | 1150 | 1300 |
ƽ�ⳣ�� | 4.0 | 3.7 | 3.5 |
A.��H��0
B.1000��ʱ���ڹ̶�������ܱ������У�ijʱ�̲��������ϵ�У�CO��CO2�����ʵ����ֱ�Ϊ0.5 mol��1.8 mol����ʱ��Ӧ������Ӧ�������
C.�����������䣬��ƽ����ϵ����CO2���壬Kֵ��С
D.�����������䣬�����¶ȣ��������CO��ƽ��ת����