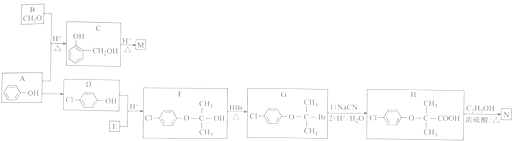
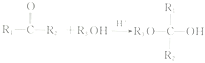��Ŀ����
����Ŀ��25 ��Cʱ����1.0 L WmolL-1��CH3COOH��Һ��0.1 mol NaOH�����ϣ���ַ�Ӧ������Һ��ͨ���ӣ���HC1�����NaOH���壬��ҺpH�����HC1��NaOH�����ʵ����ı仯��ͼ��ʾ������������ȷ����
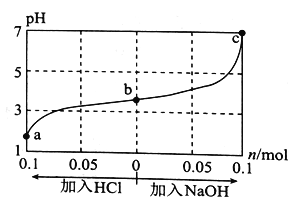
A. a��b��c��Ӧ�Ļ��Һ�У�ˮ�ĵ���̶��ɴ�С��˳���ǣ�a>b>c
B. c����Һ�У�c(Na+)>c(CH3COO-)
C. ����NaOH�����У�![]() ��ֵ��С
��ֵ��С
D. ����������仯����25 ��CʱCH3COOH�ĵ��볣�� K=![]() ��10-7 molL-1
��10-7 molL-1
���𰸡�D
��������1.0LW molL-1 CH3COOH��Һ��0.1mol NaOH�����ϣ���Ϻ���Һ��pH��5�������ԣ�˵�������������Һ������ΪCH3COOH��CH3COONa��������ʱ��CH3COONa�����ᷴӦ����CH3COOH����NaOHʱ��NaOH��CH3COOH��Ӧ����CH3COONa��A����Һ���������������ӻ����������ӵ�Ũ��Խ��ˮ�ĵ���̶�ԽС��a��b��c������Һ��������Ũ�����μ�С��ˮ�ĵ���̶���������ˮ�ĵ���̶��ɴ�С��˳�����c��b��a��A����B��c��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊ��c��Na+��+c��H+��=c��OH-��+c��CH3COO-��������c��Na+��=c��CH3COO-����B����C������NaOH�����У�c��Na+����c��OH-������c��Na+�����������c��CH3COO-�����������Լ���NaOH�����У�![]() ����C����D��pH=7ʱ��c��H+��=10-7molL-1��c��Na+��=c��CH3COO-��=0.2mol/L��Ka=c(CH3COO)��c(H+)/c(CH3COOH)=
����C����D��pH=7ʱ��c��H+��=10-7molL-1��c��Na+��=c��CH3COO-��=0.2mol/L��Ka=c(CH3COO)��c(H+)/c(CH3COOH)= ![]() ��10-7 molL-1��D��ȷ����ѡD��
��10-7 molL-1��D��ȷ����ѡD��