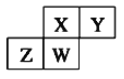
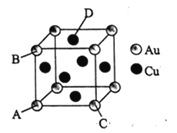
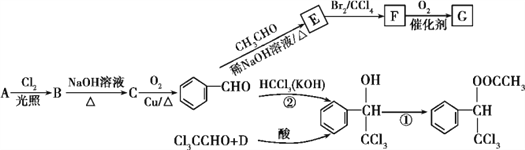
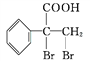
��Ŀ����
����Ŀ����ͼ�е�ʵ��װ�ÿ�������ȡ��Ȳ�����������գ�
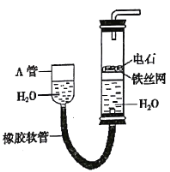
��1��ͼ�У�A�ܵ�������___����ȡ��Ȳ�Ļ�ѧ����ʽ��___��
��2����Ȳȼ��ʱ��ʵ��������___��ȼ��ʱ�������¶ȷdz��ߣ�����Ϊ����Ȳ��������Ȳ�����;��___��
��3��ʹ����ͼװ�÷�Ӧʱ����Ӧ�ٶ����ɽϿ죬��˿��ԶԷ�Ӧ����е�������������ķ���Ϊ��___��
��4����Ȳ���屾����ɫ��ζ���壬��ͨ����ʯ�Ʊ���������Ȳ���г�ζ��Ϊ�˳�ȥ������Ȳ�����е����ʣ�Ӧ����ͨ��___����Һ(ѡ������������������������)��
���𰸡�����Һ����Ʒ�Ӧ��ʼ��ֹͣ CaC2+2H2O��Ca(OH)2+CH![]() CH�� ���������棬������Ũ��ĺ��� �и���� ��ˮ��Ϊ����ʳ��ˮ ��
CH�� ���������棬������Ũ��ĺ��� �и���� ��ˮ��Ϊ����ʳ��ˮ ��
��������
��1��ͨ��ͼ��A�ܣ����Կ��Ʒ�Ӧ�Ŀ�ʼ��ֹͣ��ʵ����ͨ����ʯ��ˮ�ķ�Ӧ��ȡ��Ȳ��
��2��������Ȳ�к�̼����ȼ�շ�����������жϣ�
��3����ˮ��Ϊ����ʳ��ˮ��������Ȳ��ˮ��Ӧ�����ʣ�
��4��ʵ�����õ�ʯ�ͱ���ʳ��ˮ��Ӧ�Ʊ���Ȳ�����ɵ������к���H2S��PH3�����塣
��1��ͼʾװ�ÿ�ͨ������A�ܵĸ߶ȣ����Ʒ�Ӧ�ķ�����ֹͣ����A����ߣ��ҹ���ˮ�����������ʯ�Ӵ�������Ӧ����A�ܽ��ͣ��ҹ���ˮ���½���ˮ���ʯ����Ӵ�����Ӧֹͣ������ȡ��Ȳ�Ļ�ѧ��Ӧ����ʽΪCaC2+2H2O��Ca(OH)2+CH![]() CH����
CH����
��2����Ȳȼ��ʱ��ʵ�����������������棬������Ũ��ĺ��̡�ȼ��ʱ�������¶ȷdz��ߣ�����Ϊ����Ȳ��������Ȳ�����;���и������
��3��ʹ����ͼװ�÷�Ӧʱ����Ӧ�ٶ����ɽϿ죬��˿��ԶԷ�Ӧ����е�������ˮ��Ϊ����ʳ��ˮ��
��4��ʵ�����õ�ʯ�ͱ���ʳ��ˮ��Ӧ�Ʊ���Ȳ�����ɵ������к���H2S��PH3��������г�ζ��Ϊ�˳�ȥ������Ȳ�����е����ʣ�Ӧ����ͨ��������Һ��

����Ŀ������ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ�����һ�µ���
ѡ�� | ʵ����������� | Ԥ��ʵ��Ŀ�Ļ���� |
A | ����֧ʢ��KI3����Һ���Թ��У��ֱ�μӵ�����Һ��AgNO3��Һ��ǰ����Һ�����������л�ɫ���� | KI3��Һ�д���ƽ��:I3- |
B | ��1 mLŨ�Ⱦ�Ϊ0.05 mol��L-l NaCl��NaI�Ļ����Һ�еμ�2��0.01 mol��L-l AgNO3��Һ���������ʻ�ɫ | Ksp(AgCl)<Ksp(AgI) |
C | �����£��� pH ��ֽ�ֱ�ⶨŨ��Ϊ 0.1mol/L NaClO��Һ��0.1mol/L CH3COONa��Һ��pH | �Ƚ�HC1O��CH3COOH������ǿ�� |
D | Ũ�������Ҵ�180�湲�ȣ��Ƶõ�����ͨ������KMnO4 ��Һ����Һ��ɫ��ȥ | �Ƶõ�����Ϊ��ϩ |
A.AB.BC.CD.D