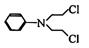
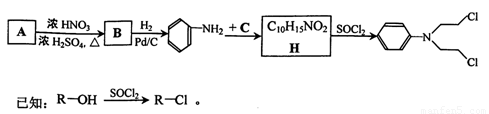
��Ŀ����
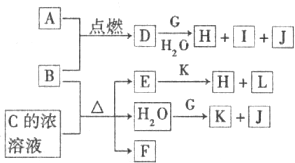
A��GΪ��ѧ�����Ļ��������֮������ͼ
��ʾ��ת����ϵ����Ӧ���������ֲ�������ȥ����AΪ��ɫ
��ĩ����H��C��O��Cu����Ԫ�ء�������DΪ��ɫ��ζ
���壬BΪ��ɫ��ĩ��E�ܷ���������Ӧ����ش�
��1��D��G��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��

��2��F��һ�����еĹ����ŵ�����Ϊ_______________________��
��3��ij����С��ͬѧ���������ʵ��װ�ã�ͨ���ⶨijЩװ�����Լ��������仯��̽��A�и�Ԫ�ص�������ϵ��
�� Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�
�� ��װ���й��������Ŀ����_________________________________________________________��
��װ����ҩƷ������Ϊ_______________________________��ʵ��ʱ����ҩƷδ�����Ա仯��֤��_______________________________________________________________________________��
�� ����ж�A����ȫ�ֽ⣿____________________________________________________________��
�� ����ȷ�IJⶨ�ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0 g��Ϊ6.0 g��װ��������0.90 g��д��A�Ļ�ѧʽ����ʾΪ��ʽ�Σ���__________________________________________
 ��1��2Na2O2+2CO2=2Na2CO3+O2��2�֣��� ��2���ǻ� ��2�֣�
��1��2Na2O2+2CO2=2Na2CO3+O2��2�֣��� ��2���ǻ� ��2�֣�
![]() ��3����
��3����
��
����1�֡���2�֣�
�� ��A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�У���ˮ����ͭ��
A�ֽ������ˮ����ȫ����Ũ�������� ����1�֡���3�֣�
�� �������μ��ȡ���������ȴ��������װ�õ����������������0.1 g ��2�֣�
�� CuCO3��2Cu(OH)2��Cu3(OH)4CO3 ��3�֣�
����:

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д� ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����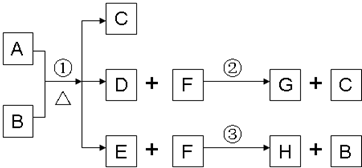

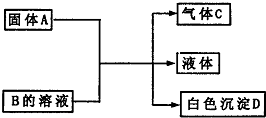 A��B��C��DΪ��ѧ��ѧ���������ʣ����Ǽ�ķ�Ӧ��ϵ��ͼ��ʾ��
A��B��C��DΪ��ѧ��ѧ���������ʣ����Ǽ�ķ�Ӧ��ϵ��ͼ��ʾ��