��Ŀ����
��12�֣�ѧϰС��̽���кͷ�Ӧ��
I ȡ20mL l mol/L��KOH��Һ���μӷ�̪��Һ
II ��I����Һ����μ���ijŨ�ȵ�H2SO4��Һ����10mLʱ���պ���ȫ��Ӧ
III ��ȡ20mL l mol/L��KOH��Һ��������II��ͬŨ�ȵ�H2SO4��Һ20mL
�� ����III��������Һ���õ���ɫ�����
V ���������ù������ȫ����ˮ������0.1mol/L Ba��OH��2��Һ���������
��1��I��ʵ���������� ��
��2��II�з�Ӧ�����ӷ���ʽ�� ������H2SO4��Һ�����ʵ���Ũ���� ��
��3��III��������Һ��������ӵ����ʵ���Ũ�ȷֱ��� ��
��4��V�еõ���������ʱ��Ӧ�����ӷ���ʽ�� ���������Ba��OH��2��Һ������� ��
I ȡ20mL l mol/L��KOH��Һ���μӷ�̪��Һ
II ��I����Һ����μ���ijŨ�ȵ�H2SO4��Һ����10mLʱ���պ���ȫ��Ӧ
III ��ȡ20mL l mol/L��KOH��Һ��������II��ͬŨ�ȵ�H2SO4��Һ20mL
�� ����III��������Һ���õ���ɫ�����
V ���������ù������ȫ����ˮ������0.1mol/L Ba��OH��2��Һ���������
��1��I��ʵ���������� ��
��2��II�з�Ӧ�����ӷ���ʽ�� ������H2SO4��Һ�����ʵ���Ũ���� ��
��3��III��������Һ��������ӵ����ʵ���Ũ�ȷֱ��� ��
��4��V�еõ���������ʱ��Ӧ�����ӷ���ʽ�� ���������Ba��OH��2��Һ������� ��
��1���������� ��Һ��죬��ҺΪ���� ��
��2�����ӷ���ʽ��H+��OH-=H2O
H2SO4�����ʵ���Ũ���� 1mol/L ��
��3�������ӵ����ʵ���Ũ�ȷֱ���c��K+��=0.5mol/L��c��H+��="0.5mol/L"
��4�����ӷ���ʽ��H+��SO42-��OH-��Ba2+=BaSO4��+H2O��
�����200mL
��2�����ӷ���ʽ��H+��OH-=H2O
H2SO4�����ʵ���Ũ���� 1mol/L ��
��3�������ӵ����ʵ���Ũ�ȷֱ���c��K+��=0.5mol/L��c��H+��="0.5mol/L"
��4�����ӷ���ʽ��H+��SO42-��OH-��Ba2+=BaSO4��+H2O��
�����200mL
��1��20mL l mol/L��KOH��Һ���μӷ�̪��Һ���ɹ۲쵽��Һ��ɺ�ɫ������ҺΪ���ԣ�
��2������кͷ�Ӧ�������ӷ�Ӧ����ʽΪ��H+��OH-=H2O�����ݷ���ʽ���������H2SO4��Һ�����ʵ���Ũ����1mol/L��
��3������������ԣ�III��������Һ��������ӵ����ʵ���Ũ�ȷֱ��ǣ�c��K+��=0.5mol/L��c��H+��=0.5mol/L��
��4��V�еõ���������ʱ��Ӧ�����ӷ���ʽ�ǣ�H+��SO42-��OH-��Ba2+=BaSO4��+H2O���������Ba��OH��2��Һ�������200mL��
��2������кͷ�Ӧ�������ӷ�Ӧ����ʽΪ��H+��OH-=H2O�����ݷ���ʽ���������H2SO4��Һ�����ʵ���Ũ����1mol/L��
��3������������ԣ�III��������Һ��������ӵ����ʵ���Ũ�ȷֱ��ǣ�c��K+��=0.5mol/L��c��H+��=0.5mol/L��
��4��V�еõ���������ʱ��Ӧ�����ӷ���ʽ�ǣ�H+��SO42-��OH-��Ba2+=BaSO4��+H2O���������Ba��OH��2��Һ�������200mL��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
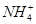 ��
��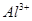 ��
��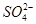 ����
����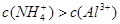
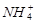 ��
��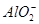 ��
��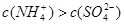
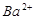 ��
��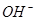 ����
����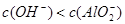
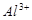 ��
��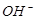 ����
����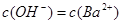
 )2
)2 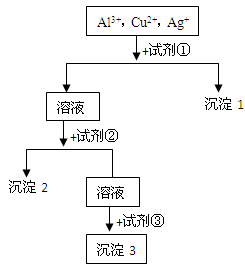
 3����ȡ������Һ��������������ͭ�ۣ�ͭ���ܽ⣬��������ð��������Һ�п϶����ڵ��������� ��
3����ȡ������Һ��������������ͭ�ۣ�ͭ���ܽ⣬��������ð��������Һ�п϶����ڵ��������� ��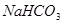 ����
����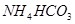 ����
���� ����HF����
����HF����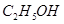 ����
����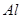 ����ʳ��ˮ����
����ʳ��ˮ����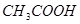
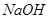 ��Һ��Ӧ����_____________��
��Һ��Ӧ����_____________��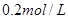 ��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֣�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ
��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֣�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ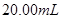 ����
���� ��Һ����μ���
��Һ����μ��� _______________��
_______________��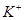 ��
��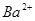 ��
��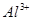 ��
��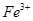
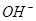 ��
�� ��
��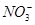 ��
��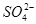
 ���£�
���£�