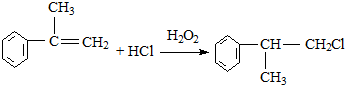��Ŀ����
�ش��������⣺
��1������������Zn�۷ֱ�Ͷ�룺�Թ�A��10mL 0.1mol?L-1 HCl���Թ�B��10mL 0.1mol?L-1�����У���ʼʱ����Ӧ����A
��2����������Zn�۷ֱ�Ͷ��pH=1�����Ϊ10mL���Թ�A��������Թ�B�������У���ʼʱ����Ӧ����A
��3��������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ���Һ��
��4��pH=3�Ĵ����pH=11������������Һ�������Ϻ���Һ��
��5���Ȼ���ˮ��Һ��
��6��������������Һʱ��Ϊ�˷�ֹ����ˮ�⣬���Լ���������
��1������������Zn�۷ֱ�Ͷ�룺�Թ�A��10mL 0.1mol?L-1 HCl���Թ�B��10mL 0.1mol?L-1�����У���ʼʱ����Ӧ����A
��
��
B�����=����ͬ������Zn����������H2�����A=
=
B����2����������Zn�۷ֱ�Ͷ��pH=1�����Ϊ10mL���Թ�A��������Թ�B�������У���ʼʱ����Ӧ����A
=
=
B�����=����ͬ������Zn����������H2�����A��
��
B����3��������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ���Һ��
����
����
������ԡ��������ԡ����ԡ�����ͬ������4��pH=3�Ĵ����pH=11������������Һ�������Ϻ���Һ��
����
����
����5���Ȼ���ˮ��Һ��
����
����
�ԣ�ԭ���ǣ������ӷ���ʽ��ʾ����Al3++3H2O?Al��OH��3+3H+
Al3++3H2O?Al��OH��3+3H+
����AlCl3��Һ���ɣ����գ����õ�����Ҫ���������Al2O3
Al2O3
����6��������������Һʱ��Ϊ�˷�ֹ����ˮ�⣬���Լ���������
NaOH
NaOH
����������1����2������Ϊ���ᣬ������ȫ���룬�ɸ��ݴ������������ʵ����Լ�pH�Ĺ�ϵ�ж���ҺŨ�ȴ�С���Դ˽����⣻
��3����������ǿ������������Һ�ʼ��ԣ�
��4��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ��������
��5���Ȼ�����ǿ�������Σ��Ȼ���ˮ�����Һ�����ԣ�
��6��������ǿ�������Σ�����Һ�д���ˮ��ƽ�⣬��ƽ���ƶ��ĽǶȷ����������⣮
��3����������ǿ������������Һ�ʼ��ԣ�
��4��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ��������
��5���Ȼ�����ǿ�������Σ��Ȼ���ˮ�����Һ�����ԣ�
��6��������ǿ�������Σ�����Һ�д���ˮ��ƽ�⣬��ƽ���ƶ��ĽǶȷ����������⣮
����⣺��1��HCl�ʹ���Ũ����ͬ�����ڴ���Ϊ���ᣬ��������Һ��������Ũ�ȴ��ڴ���������Ũ�ȣ���ʼʱ����Ӧ����A��B���������ʵ�����ͬ����Zn����������H2�������ͬ���ʴ�Ϊ������=��
��2��pH��ͬ������ʹ��ᣬ���ڴ���Ϊ���ᣬ������ȫ���룬������Ũ�ȴ��������Ũ�ȣ���ʼʱ����Ӧ����A=B����Zn����������H2�����A��B��
�ʴ�Ϊ��=������
��3������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ���ǡ�÷�Ӧ���ɴ����ƣ���������ǿ������������Һ�ʼ��ԣ��ʴ�Ϊ�����ԣ�
��4��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ��������Ϊ����ʹ����ƵĻ����Һ������Һ�����ԣ�
�ʴ�Ϊ���
��5���Ȼ���Ϊǿ�������Σ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O?Al��OH��3+3H+��ˮ�����Һ�����ԣ�����ʱ�ٽ�ˮ�⣬����Al��OH��3��Al��OH��3���ȶ�������ʱ�ֽ�����Al2O3��
�ʴ�Ϊ�����ԣ� Al3++3H2O?Al��OH��3+3H+��Al2O3��
��6������Ϊǿ�������Σ�����Һ�д���ˮ��ƽ�⣺S2-+H2O?HS-+OH-��Ϊ�˷�ֹ����ˮ�⣬���Լ�������
NaOH��ʹƽ�����淴Ӧ�����ƶ����Ӷ�����ˮ�⣬�ʴ�Ϊ��NaOH��
��2��pH��ͬ������ʹ��ᣬ���ڴ���Ϊ���ᣬ������ȫ���룬������Ũ�ȴ��������Ũ�ȣ���ʼʱ����Ӧ����A=B����Zn����������H2�����A��B��
�ʴ�Ϊ��=������
��3������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ���ǡ�÷�Ӧ���ɴ����ƣ���������ǿ������������Һ�ʼ��ԣ��ʴ�Ϊ�����ԣ�
��4��pH=3�Ĵ��ᣬ��Ũ�ȴ���0.001mol/L��pH=11���������ƣ���Ũ�ȵ���0.001mol/L���������Ϻ��������Ϊ����ʹ����ƵĻ����Һ������Һ�����ԣ�
�ʴ�Ϊ���
��5���Ȼ���Ϊǿ�������Σ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O?Al��OH��3+3H+��ˮ�����Һ�����ԣ�����ʱ�ٽ�ˮ�⣬����Al��OH��3��Al��OH��3���ȶ�������ʱ�ֽ�����Al2O3��
�ʴ�Ϊ�����ԣ� Al3++3H2O?Al��OH��3+3H+��Al2O3��
��6������Ϊǿ�������Σ�����Һ�д���ˮ��ƽ�⣺S2-+H2O?HS-+OH-��Ϊ�˷�ֹ����ˮ�⣬���Լ�������
NaOH��ʹƽ�����淴Ӧ�����ƶ����Ӷ�����ˮ�⣬�ʴ�Ϊ��NaOH��
���������⿼����������ʵĵ��롢����ˮ���֪ʶ�㣬��ȷ��ͬ����ͬһ������Ӧ����������Ũ�ȳ����ȣ�ע��������������ʱ������������Ϊ�״��㣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�


 ��XΪ±��ԭ�ӣ�
��XΪ±��ԭ�ӣ� �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�