��Ŀ����
7��ʵ������ȼ�շ��ⶨij��aһ������X��CxHyOzNp���ķ�����ɣ�ȡ1.67g X���ڴ����г��ȼ�գ�����CO2��H2O��N2��������ͼ��ʾװ�ý���ʵ�飨����̨�����С��ƾ��Ƶ�δ����������ش��й����⣺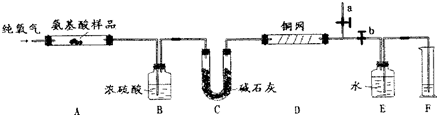
��1��ʵ�鿪ʼʱ�����ȴ�ֹˮ��a���ر�ֹˮ��b��ͨһ��ʱ��Ĵ�������������Ŀ����
��װ���е�N2�ž�֮������ر�ֹˮ��a����ֹˮ��b��
��2������װ������Ҫ���ȵ��У���װ�ô��ţ�A��D������ʱӦ�ȵ�ȼD���ľƾ��ƣ�
��3����ƽװ��A�з�����Ӧ�Ļ�ѧ����ʽ
CxHyOzNp+��x+$\frac{y}{4}$-$\frac{z}{2}$��+O2��xCO2+$\frac{y}{2}$H2O+$\frac{p}{2}$N2
��4��װ��D������������δ��Ӧ��O2����֤�����ռ����������Ƿ�Ӧ���ɵ�N2��
��5��ʵ������
��B��Ũ��������0.81g��C�м�ʯ������3.52g��F���ռ���112mL����״�������壮
��x����Է�������Ϊ167������ͨ�������Dzⶨ�ģ���һ���ִ���ѧ��������ͨ������ȷ�����л���X�ķ���ʽC8H9NO3��
���� ��1������װ�õĿ����к���N2��
��2�����ݦ�-��������ڴ����г��ȼ�գ�����ͭ���������ڼ��������·�Ӧ��ȥδ��Ӧ������������ʱӦ�ȳ�ȥ�������Է�ֹӰ��N2������IJⶨ��
��3������ƽ̼ԭ�ӡ���ԭ�ӡ���ԭ�����������ƽ��ԭ�ӣ�����ƽ��ԭ��ʱע���л����е���ԭ�ӣ����ԭ���غ���ƽ��д��
��4������ͭ���������ڼ��������·�Ӧ��ȥδ��Ӧ��������
��5��������Է������������������ⶨ�����ݼ�ʯ��������������ȷ��CO2�����������C�����������ʵ���������Ũ��������������ȷ��ˮ�����������H�����������ʵ��������ݵ������������������������ʵ��������������غ㣬����������������ʵ������Դ˸�����л���X�ķ���ʽ��
��� �⣺��1������װ�õĿ����к���N2���轫װ���е�N2�ž���
�ʴ�Ϊ����װ���е�N2�ž���a��b��
��2�����-��������ڴ����г��ȼ�գ���ͭ���������ڼ��������·�Ӧ��ȥδ��Ӧ����������A��D����Ҫ���ȣ������ʱӦ�ȳ�ȥ�������Է�ֹӰ��N2������IJⶨ��
�ʴ�Ϊ��A��D��D��
��3������ԭ���غ㣬����ƽ̼���⡢��ԭ�ӣ�����ƽ��ԭ�ӣ�ע���л����е���ԭ�ӣ���ƽ��д�õ��Ļ�ѧ����ʽΪ��CxHyOzNp+��x+$\frac{y}{4}$-$\frac{z}{2}$��O2��xCO2+$\frac{y}{2}$H2O+$\frac{p}{2}$N2��
�ʴ�Ϊ��CxHyOzNp+��x+$\frac{y}{4}$-$\frac{z}{2}$��O2��xCO2+$\frac{y}{2}$H2O+$\frac{p}{2}$N2��
��4������ͭ���������ڼ��������·�Ӧ��ȥδ��Ӧ��������
�ʴ𰸣�����δ��Ӧ��O2����֤�����ռ����������Ƿ�Ӧ���ɵ�N2��
��5����ⶨ��Է�������������������
���ʯ������������ΪCO2��������CO2������Ϊ3.52g�������ʵ���Ϊ$\frac{3.52g}{44g/mol}$=0.08mol��n��C��=0.08mol��m��C��=0.08mol��12g/mol=0.96g��
��Ũ��������������Ϊˮ��������H2O������Ϊ0.81g�������ʵ���Ϊ$\frac{0.81g}{18g/mol}$=0.045mol��n��H��=0.09mol��m��H��=0.09g��
���������ΪN2Ϊ112mL�������ʵ���Ϊ$\frac{0.112L}{22.4L/mol}$=0.005mol��n��N��=0.01mol��m��N��=0.14g��
��m��C��+m��H��+m��N��=1.19g������m��O��=1.67g-1.19g=0.48g��n��O��=$\frac{0.48g}{167g/mol}$=0.03mol��
X�����ʵ���Ϊ$\frac{1.67g}{167g/mol}$=0.01mol������0.01molX�к���C0.08molC��0.09molH��0.01molN��0.03molO�����Ը��л���X�ķ���ʽΪC8H9NO3��
�ʴ�Ϊ�������ǣ����л���X�ķ���ʽΪC8H9NO3��
���� ���⿼��ѧ��ȼ�շ�ȷ���л��������ɵ�֪ʶ������ԭ���غ㷨��ƽ��д��ѧ����ʽ�����㻯ѧʽ����ɣ�������ѧ֪ʶ������Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | F-�Ľṹʾ��ͼ��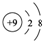 | |
| B�� | �����Ļ�ѧʽ��FeSO4•7H2O | |
| C�� | ���Ȼ�̼�ĵ���ʽ��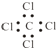 | |
| D�� | ���ں���18�����ӵ���ԭ�ӣ�${\;}_{35}^{17}$Cl |
| A�� | ������������Һ�����Ҵ���Һ����������Һ | |
| B�� | ��̼������Һ����������������� | |
| C�� | �ý�����������ˮ�Ҵ����������� | |
| D�� | �����Ը��������Һ����������ϩ |
| A�� | �ڴ���ƽ�����У����ȴ����������������£��ֽ��������ԭ�ӿ�������ij������ѭ����Ӧ | |
| B�� | ľ�Ǵ��ṹ��ʽΪ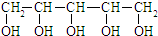 �ɴ˿ɼ���һ�������ܽ���ˮ���ɷ���������Ӧ �ɴ˿ɼ���һ�������ܽ���ˮ���ɷ���������Ӧ | |
| C�� | ���ۺ���ά�ص�ͨʽ���ǣ�C6H10O5��n�����Զ�����ͬ���칹�� | |
| D�� | ���Ҵ���C2H4�Ƿ��ȷ�Ӧ |
| ѡ�� | ���� | ������ѧ������ | �������������� |
| A | MgCl2 | ���Ӽ����Ǽ��Թ��ۼ� | ���ӻ����� |
| B | CO2 | ���Թ��ۼ� | ���ۻ����� |
| C | HCl | ���Ӽ� | ���ӻ����� |
| D | NaOH | ���Ӽ������Թ��ۼ� | ���ۻ����� |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
| A�� | H2O | B�� | MgCl2 | C�� | Na2SO4 | D�� | NH4Cl |
| A�� | Na2RO3 | B�� | Na3RO4 | C�� | NaRO3 | D�� | Na2RO4 |
| A�� | ����������Ӧ�ĵ缫 | |
| B�� | ���������ĵ缫 | |
| C�� | ������ԭ��Ӧ�ĵ缫 | |
| D�� | �븺����Ƚϣ������Խϻ��õ�һ�� |