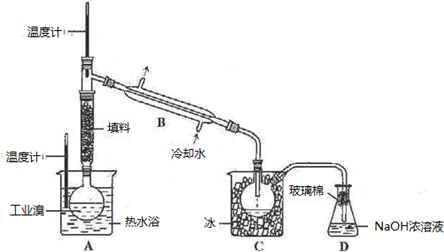
��Ŀ����
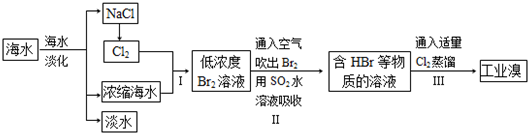
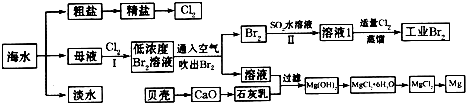
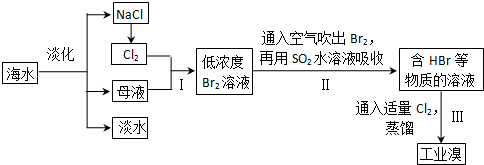
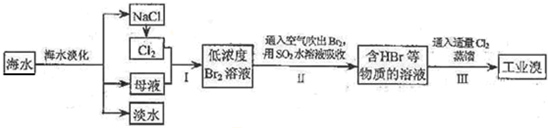
��ˮ�Ǿ����Դ���⡣��ͼ������Ӻ�ˮ��Դ��ȡijЩ��Ҫ����ԭ�ϵ�����ʾ��ͼ��

�ش��������⣺
����A��_________________(��ʵ�������������)���ú��ַ���֪����ˮɹ�εĹ�������Һ��ʳ�κ���������ߣ�________
a�������Ȼ��ƺ��� b���ⶨ��Һ�ܶ� c���۲��Ƿ��г�������
����B����������Լ��е�һ�֣�����ʵ���______��ѡ���ţ���
a������������Һ b������ʯ��ˮ c��ʯ���� d��̼������Һ
���±��ͨ��Cl2��������Ӧ�����ӷ���ʽ��____________________________������C��_______________________________��
��ͼ�����߿������̵���Ҫ������_______________________����֮Ŀ����ͬ����������ͼ�л��м�������ָ������һ��________________________��
��MgCl2ת��ΪMgʱ��õĸ���Ʒ��_____________���û�ѧ����ʽ��ʾ�����ʵ�ij����;______________________________________��
�����ᾧ��1�֣���b��2�֣� c����1�֣�
Cl2 + 2Br- �� Br2 + 2Cl-����2�֣� �ȿ���������1�֣�
����Br2��1�֣�����ˮ�����ᾧ�õ����Ρ���±��Mg(OH)2��MgCl2��Һ����д������֮һ���ɣ�1�֣�
Cl2����1�֣� Cl2 + 2NaOH �� NaCl + NaClO + H2O�����������𰸾��ɣ���2�֣�
��������
�����������1���Ӻ�ˮ�еõ����εIJ���Ӧ���������ᾧ���Ȼ��Ƶĺ����ⶨ�Ƚϸ��ӣ����۲취�ֲ�ȷ��������õİ취�Dzⶨ��Һ���ܶȣ���ѡb��
��2�����±�м���ʯ���鼴������������þ��������ѡc��
��3����ȴ���������ԣ��ܰ��������������ɵ����壬��Ӧ�ķ���ʽ��Cl2 + 2Br- �� Br2 + 2Cl-��Ҫʹ���ɵĵ������������ͨ���ȿ����������ɡ�
��3����ͼ�����߿������̵���Ҫ�����Ǹ��������塣��������ͼ���жϺ�ˮ�����ᾧ�õ����Ρ���±��Mg(OH)2��MgCl2��Һ�Ⱦ��Ǹ���������ġ�
��4��������ڵ��Ȼ�þ����ұ������þ��ͬʱ�õ��������ڹ�ҵ�����������Ʊ�Ư��Һ����Ӧ�ķ���ʽ��Cl2 + 2NaOH �� NaCl + NaClO + H2O��
���㣺���麣ˮ�ۺ�Ӧ�õ��й��жϡ������������Լ���ѡ���Լ�����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ���������У����ض�ѧ������֪ʶ�Ĺ�����ѵ����ͬʱҲע�ض�ѧ�����������������ͷ���ָ��������������ѧ������˼ά�����ͷ�ɢ˼ά����������������һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ���������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά������

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�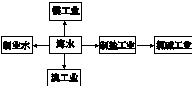
| A����ˮ�Ƶ�ˮ��Ҫ�������������������ӽ������� | B����ˮ���Ρ���չ�ȼҵ���Ƿ��������仯 | C����ˮ�����������ͨ��Cl2������������Ϊ�嵥�� | D����ҵ���õ������MgCl2�ķ�����ȡ����þ |