��Ŀ����
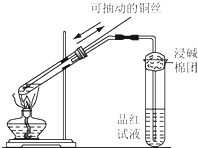 ijͬѧ����ͼ��ʾ��װ�ý���ͭ��Ũ���ᷴӦ��ʵ�飮��ش����⣮
ijͬѧ����ͼ��ʾ��װ�ý���ͭ��Ũ���ᷴӦ��ʵ�飮��ش����⣮��1��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2��
| ||
Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2��
��
| ||
��2��ʵ������У��۲쵽Ʒ����Һ
��ɫ
��ɫ
��Ϊ��һ��ȷ�ϲ����������Ƕ����������壬��Ӧ����IJ�����������ɫ���Ʒ����Һ
������ɫ���Ʒ����Һ
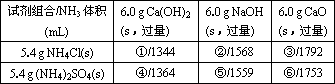
����3������������Ԥ�Ƚ�����������Ũ��Һ����������
���ն����SO2
���ն����SO2
���÷�Ӧ�����ӷ���ʽ��SO2+2OH-=SO32-+H2O
SO2+2OH-=SO32-+H2O
����4��Ϊ��ֹʵ�飬Ӧ���Ȳ�ȡ��ʵ�������
С�Ľ�ͭ˿��Ũ�����г��
С�Ľ�ͭ˿��Ũ�����г��
����������1���ڼ��ȵ������£�Ũ�����ͭ��Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2����
��2��SO2����Ư���ԣ�ʹƷ����Һ��ɫ����SO2��Ư���Dz��ȶ��ģ�
��3��SO2�Ǵ�����Ⱦ������������Ũ��Һ���ն���SO2�����ӷ���ʽ��SO2+2OH-=SO32-+H2O
��4��ʹͭ˿��Ũ������뼴����ֹʵ�飮
| ||
��2��SO2����Ư���ԣ�ʹƷ����Һ��ɫ����SO2��Ư���Dz��ȶ��ģ�
��3��SO2�Ǵ�����Ⱦ������������Ũ��Һ���ն���SO2�����ӷ���ʽ��SO2+2OH-=SO32-+H2O
��4��ʹͭ˿��Ũ������뼴����ֹʵ�飮
����⣺��1���ڼ��ȵ������£�Ũ�����ͭ��Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2�����ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+2H2O+SO2����
��2������Ļ�ԭ����SO2����Ư���ԣ���ʹƷ����Һ��ɫ����SO2��Ư���Dz��ȶ��ģ��ڼ��ȵ�����£����Իָ�����������ɫ������Ҫ��������������壬��Ҫ���ȣ���Ʒ����Һ�ָ���ɫ����֤���Ƕ������ʴ�Ϊ����ɫ��������ɫ���Ʒ����Һ��
��3��SO2�Ǵ�����Ⱦ������������Ũ��Һ���ն���SO2����ֹ��Ⱦ�����������ķ�ӦΪ��SO2+2OH-=SO32-+H2O���ʴ�Ϊ�����ն����SO2��SO2+2OH-=SO32-+H2O��
��4��ʹͭ˿��Ũ������뼴����ֹʵ�飬����Ҫ��ͭ˿��Ũ�����г�����ʴ�Ϊ��С�Ľ�ͭ˿��Ũ�����г����
| ||
| ||
��2������Ļ�ԭ����SO2����Ư���ԣ���ʹƷ����Һ��ɫ����SO2��Ư���Dz��ȶ��ģ��ڼ��ȵ�����£����Իָ�����������ɫ������Ҫ��������������壬��Ҫ���ȣ���Ʒ����Һ�ָ���ɫ����֤���Ƕ������ʴ�Ϊ����ɫ��������ɫ���Ʒ����Һ��
��3��SO2�Ǵ�����Ⱦ������������Ũ��Һ���ն���SO2����ֹ��Ⱦ�����������ķ�ӦΪ��SO2+2OH-=SO32-+H2O���ʴ�Ϊ�����ն����SO2��SO2+2OH-=SO32-+H2O��
��4��ʹͭ˿��Ũ������뼴����ֹʵ�飬����Ҫ��ͭ˿��Ũ�����г�����ʴ�Ϊ��С�Ľ�ͭ˿��Ũ�����г����
���������⿼����Ũ�����ͭ�ķ�Ӧ��������������ʼ����飬���ӷ��̵���д���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
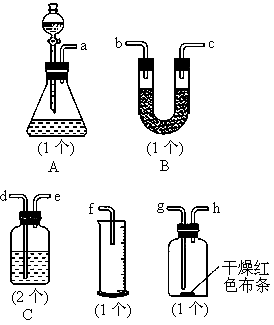
ijͬѧ����ͼ��ʾ��װ������ȡ��̽�����壨H2��O2��Cl2��SO2��NO2�������ʣ�
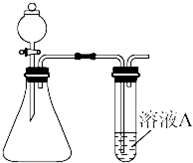 ��1��װҩƷǰ��Ӧ�Ƚ��еIJ���
��1��װҩƷǰ��Ӧ�Ƚ��еIJ���
��2������ø�װ����ȡH2��O2����ѡ�õ��Լ������ ������ţ���
��3�����������У���ͬʱ������ͼ��ʾ�����ռ�װ���ռ��������� ��
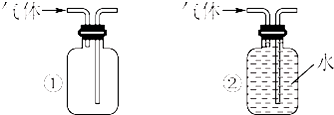
��4����ͬѧ�ֱ����ø�װ����ȡ��̽���������ʣ�ͬѧ�������������ƹ��������ᷴӦ��ȡSO2��ͬѧ���ø�����غ�������ȡ��������ش�
��д���������ƹ��������ᷴӦ��ȡSO2�Ļ�ѧ����ʽ�� ��
������ҺAΪ��ˮ��ͨ��SO2�������� ������ҺAΪ�廯����Һ��ͨ������ʱ��Ӧ�����ӷ���ʽΪ ��ʵ��֤��Br2��SO2��Cl2�������ɴ�С��˳��Ϊ ��
����ͬѧ��Ƚ�Cl2��SO2Ư�����ʵĿ�����A��Һѡȡ������ͬ����Ʒ����Һ��ͬʱ��ȡ����Cl2��SO2���۲�Ʒ����Һ��ɫ��ʱ�䣬�Ƚ���ɫ�Ŀ������÷������ڵ�ȱ��Ϊ ����ͬѧ����Ƚ�Cl2��
SO2Ư�IJ����ԣ�����Ҫ�IJ����Ϳ��ܹ۲쵽������Ϊ ��
 ��1��װҩƷǰ��Ӧ�Ƚ��еIJ���
��1��װҩƷǰ��Ӧ�Ƚ��еIJ�����2������ø�װ����ȡH2��O2����ѡ�õ��Լ������
| H2 | O2 | |
| �� | Zn��ϡ���� | KClO3��MnO2 |
| �� | Zn��ϡ���� | H2O2��Һ��MnO2 |
| �� | Zn��ϡ���� | KMnO4 |
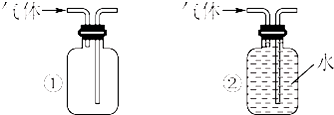
��4����ͬѧ�ֱ����ø�װ����ȡ��̽���������ʣ�ͬѧ�������������ƹ��������ᷴӦ��ȡSO2��ͬѧ���ø�����غ�������ȡ��������ش�
��д���������ƹ��������ᷴӦ��ȡSO2�Ļ�ѧ����ʽ��
������ҺAΪ��ˮ��ͨ��SO2��������
����ͬѧ��Ƚ�Cl2��SO2Ư�����ʵĿ�����A��Һѡȡ������ͬ����Ʒ����Һ��ͬʱ��ȡ����Cl2��SO2���۲�Ʒ����Һ��ɫ��ʱ�䣬�Ƚ���ɫ�Ŀ������÷������ڵ�ȱ��Ϊ
SO2Ư�IJ����ԣ�����Ҫ�IJ����Ϳ��ܹ۲쵽������Ϊ