��Ŀ����
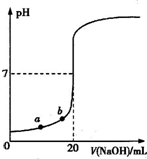
��25mL����������Һ����μ���0.2mol?L-1������Һ���ζ�������ͼ��ʾ��
��1��д������������Һ�������Һ��Ӧ�����ӷ���ʽ��______��
��2��������������Һ�����ʵ���Ũ��Ϊ______mol?L-1��
��3����B�㣬a______12.5mL�����������������=������ͬ�������������ȵ��������ƺʹ�����Һ��϶���ǡ�ó����ԣ�����ǰc��NaOH��______c��CH3COOH�������ǰ����c��H+���ͼ���c��OH-���Ĺ�ϵ��c��H+��______c��OH-����
��4����D�㣬��Һ������Ũ�ȴ�С��ϵΪ��______��

��1��д������������Һ�������Һ��Ӧ�����ӷ���ʽ��______��
��2��������������Һ�����ʵ���Ũ��Ϊ______mol?L-1��
��3����B�㣬a______12.5mL�����������������=������ͬ�������������ȵ��������ƺʹ�����Һ��϶���ǡ�ó����ԣ�����ǰc��NaOH��______c��CH3COOH�������ǰ����c��H+���ͼ���c��OH-���Ĺ�ϵ��c��H+��______c��OH-����
��4����D�㣬��Һ������Ũ�ȴ�С��ϵΪ��______��

��1����Ӧ���ɴ����ƺ�ˮ�������ˮ�����ӷ�Ӧ�б�����ѧʽ�������ӷ�ӦΪOH-+CH3COOH�TCH3COO-+H2O���ʴ�Ϊ��OH-+CH3COOH�TCH3COO-+H2O��
��2���ɿ�ʼNaOH��Һ��pH=13��c��OH-��=c��NaOH��=0.1mol/L���ʴ�Ϊ��0.1��
��3��B��pH=7��a=12.5mLʱǡ�����ɴ����ƣ���Һ�Լ��ԣ������Ӧ����ʹpH=7����a��12.5mL���������ȵ��������ƺʹ�����Һ��϶���ǡ�ó����ԣ�Ũ����ͬʱ��Һ�Լ��ԣ������Ũ�ȴ�c��NaOH����c��CH3COOH�����ֻ��ǰ����ȫ���룬�������ȫ���룬����ǰ����c��H+���ͼ���c��OH-���Ĺ�ϵΪc��H+����c��OH-�����ʴ�Ϊ��������������
��4��D�㣬��Һ�����ԣ�Ϊ�����ƺʹ���Ļ����Һ���������Ϊ����������Ũ�ȴ�С��ϵΪc��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
��2���ɿ�ʼNaOH��Һ��pH=13��c��OH-��=c��NaOH��=0.1mol/L���ʴ�Ϊ��0.1��
��3��B��pH=7��a=12.5mLʱǡ�����ɴ����ƣ���Һ�Լ��ԣ������Ӧ����ʹpH=7����a��12.5mL���������ȵ��������ƺʹ�����Һ��϶���ǡ�ó����ԣ�Ũ����ͬʱ��Һ�Լ��ԣ������Ũ�ȴ�c��NaOH����c��CH3COOH�����ֻ��ǰ����ȫ���룬�������ȫ���룬����ǰ����c��H+���ͼ���c��OH-���Ĺ�ϵΪc��H+����c��OH-�����ʴ�Ϊ��������������
��4��D�㣬��Һ�����ԣ�Ϊ�����ƺʹ���Ļ����Һ���������Ϊ����������Ũ�ȴ�С��ϵΪc��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����

��ϰ��ϵ�д�
�����Ŀ