��Ŀ����
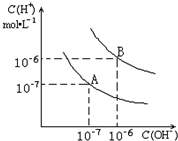
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��1��25��ʱ����pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=7����NaOH��Һ��H2SO4��Һ�������Ϊ______��
��2��95��ʱ����pH=a��ijǿ����Һ��pH=b��ijǿ����Һ�������Ϻ���Һ�����ԣ���a��bӦ����Ĺ�ϵ��______��
��3������B��Ӧ�¶��£�pH=2��ij��HA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5���������ԭ��______��
��1��25��ʱ����pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=7����NaOH��Һ��H2SO4��Һ�������Ϊ______��
��2��95��ʱ����pH=a��ijǿ����Һ��pH=b��ijǿ����Һ�������Ϻ���Һ�����ԣ���a��bӦ����Ĺ�ϵ��______��
��3������B��Ӧ�¶��£�pH=2��ij��HA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5���������ԭ��______��

��1����25��ʱ���û����Һ��pH=7����Һ�����Լ����ǡ���кͣ���n��OH-��=n��H+������V��NaOH��?10-5 mol?L-1=V��H2SO4��?10-4 mol?L-1����V��NaOH����V��H2SO4��=10��1���ʴ�Ϊ��10��1��
��2��Ҫע�����95��Cʱ��ˮ�����ӻ�Ϊ10-12�����ᡢ��Ũ�����ʱpH���ᣩ+pH���=12����a+b=12���ʴ�Ϊ��a+b=12����10-a=10b-12����
��3��������B��Ӧ�¶��£���pH���ᣩ+pH���=12���ɵ��������Һ��c��H+��=c��OH-��������ǿ������Һ�������Ϻ���Һ�����ԣ��ֻ����Һ��pH=5�����������Ϻ���Һ�����ԣ�˵��H+��OH-��ȫ��Ӧ�������µ�H+�������������������HA�����ᣬ
�ʴ�Ϊ������B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
��2��Ҫע�����95��Cʱ��ˮ�����ӻ�Ϊ10-12�����ᡢ��Ũ�����ʱpH���ᣩ+pH���=12����a+b=12���ʴ�Ϊ��a+b=12����10-a=10b-12����
��3��������B��Ӧ�¶��£���pH���ᣩ+pH���=12���ɵ��������Һ��c��H+��=c��OH-��������ǿ������Һ�������Ϻ���Һ�����ԣ��ֻ����Һ��pH=5�����������Ϻ���Һ�����ԣ�˵��H+��OH-��ȫ��Ӧ�������µ�H+�������������������HA�����ᣬ
�ʴ�Ϊ������B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��

��ϰ��ϵ�д�
�����Ŀ

