��Ŀ����
19����ͼ�ǽ���ͭ��Ũ���ᷴӦ�����в�������װ�ã���1��A���ڷ�����Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��2����Ӧ��A�Թ��е�Һ��������ˮϡ�ͺ�����ɫ��B��Һ��Ʒ����Һ����ɫ����Ϊ��ɫ��
��3��C�Թ�������������Һ�����������ն����SO2����ֹ������Ⱦ�����ӷ�Ӧ����ʽΪSO2+2OH-=SO32-+H2O��
��4��Ϊ��ʹ��Ӧ��ͣ���ã����Խ�װ�������Ľ�����ͭƬ��Ϊ�ɳ鶯��ͭ˿��
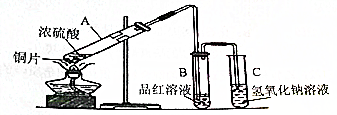
���� ��1��A�Թ���ͭ��Ũ������ȷ�Ӧ��������ͭ�����������ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��2��ͭ��Ũ���ᷴӦ����������ͭ����Ӧ�����Һϡ�ͺ���Һ��ʾ��ɫ����Ӧ���ɵĶ��������������Ư���ԣ��ܹ�ʹƷ����Һ��ɫ��
��3�����������ж�����Ҫʹ������������Һ����δ��Ӧ�Ķ�������������������������Һ��Ӧ�����������ƺ�ˮ���ݴ�д����Ӧ�����ӷ���ʽ��
��4��ͨ������ͭƬ��Ϊ�ɳ鶯��ͭ˿�����Կ��Ʒ�Ӧ�Ŀ�ʼ��ֹͣ��
��� �⣺��1��ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��2����Ӧ��A�Թ��е�Һ���к�������ͭ��������ˮϡ�ͺ�����ɫ��ͭ��Ũ���ᷴӦ�����˾���Ư���ԵĶ�����������B�Թ���Ʒ����Һ��ɫ�����ɺ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ��������ɫ��
��3����������Ϊ�ж����壬��Ҫ����β�����գ�C������������Һ�����������ն����SO2����ֹ������Ⱦ����������������������Һ��Ӧ�����ӷ���ʽΪ��SO2+2OH-=SO32-+H2O��
�ʴ�Ϊ�����ն����SO2����ֹ������Ⱦ��SO2+2OH-=SO32-+H2O��
��4����ͭƬ��Ϊ�ɳ鶯��ͭ˿��ͨ������ͭ˿�ĸ߶ȣ����Կ��Ʋμӷ�Ӧ��ͭ��Ũ����ĽӴ�������Ӷ���֪��������������������ٶ����������Ⱦ���÷���������ɫ��ѧ�����
�ʴ�Ϊ����ͭƬ��Ϊ�ɳ鶯��ͭ˿��
���� ���⿼����Ũ��������ʡ�������������ʼ����飬��Ŀ�Ѷ��еȣ�ע������ͭ��Ũ���ᷴӦԭ��������������������ʼ����鷽������ȷ����ʵ�鷽����������۵�ԭ��

 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�| A�� | �������غ㶨�ɣ���Ӧ���������һ������������������� | |
| B�� | ֻҪ�������ı仯��һ�������˻�ѧ��Ӧ | |
| C�� | ���еĻ�ѧ��Ӧ���������仯 | |
| D�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ |
| A�� | C60 | B�� | CaCl2 | C�� | KF | D�� | NH4Cl |
| A�� | ������������һ����CH2 ԭ���ŵ��л�����ͬϵ�� | |
| B�� | �����Ԫ������������ͬ������ͬһ���� | |
| C�� | ����ʽ��ͬ���ṹ��ͬ���л��ﲻһ����ͬ���칹�� | |
| D�� | ��Ϊͬϵ����л�������ӽṹ��Ȼ���� |
| A�� | ���¶ȸ���25�� | |
| B�� | c��H+��=c��OH-��+c��SO42-�� | |
| C�� | ˮ���������c��H+��=1��10-10mol•L-1 | |
| D�� | ���¶��¼�������pH=12��NaOH��Һ��ʹ��Ӧ�����Һǡ�������� |
| A�� | ԭ�Ӱ뾶��X��Y��Z��W | B�� | �����ԣ�X��Y����ԭ�ԣ�W 2-��Z- | ||
| C�� | ԭ��������������Y��X��Z��W | D�� | ԭ��������Y��X��Z��W |
| A�� | ��������Һ������ȩ��ȩ����CH3CHO+2Ag��NH3��2++2OH-$\stackrel{ˮԡ����}{��}$CH3COO-+NH4++3NH3+2Ag��+H2O | |
| B�� | ��CH2BrCOOH�м�������������������Һ�����ȣ�CH2BrCOOH+OH-��$\stackrel{��}{��}$ CH2BrCOO-+H2O | |
| C�� | ϡHNO3ϴ���Թ��е�������3Ag+NO3-+4H+=3Ag++NO��+2H2O | |
| D�� | ������Һ��ͨ��������CO2��CO2+H2O+2C6H5O-��2C6H5OH+2CO32- |
| A�� | E��3s����E��3p����E��3d�� | B�� | E��1s����E��2s����E��3s�� | C�� | E��4f����E��3d����E��4s�� | D�� | E��5s����E��4s����E��4f�� |
| A�� | ���Ṥҵ�У�����O2��Ũ�����������SO2��ת���� | |
| B�� | ��FeS����Ͷ�뵽����Cu2+�ķ�ˮ���Գ�ȥCu2+ | |
| C�� | ѡ����ʵĴ�������߹�ҵ�ϳɰ�������Ч�� | |
| D�� | ���з�̪��CH3COONa��Һ�����Ⱥ���ɫ���� |