��Ŀ����
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��1���ձ���������ֽ����������______��
��2����Ũ�������������Һ��������ʵ�飬��õ��к��ȵ���ֵ��______�����ƫ����ƫС��������Ӱ�족����
��3����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����______
A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C������ʵ��ĵ������½ϸ�
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ
E������ȡ����ʱ���Ӽ���
F�����ձ��ĸǰ��м�С��̫��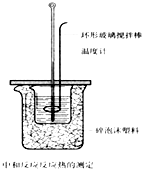
��1���ձ���������ֽ����������______��
��2����Ũ�������������Һ��������ʵ�飬��õ��к��ȵ���ֵ��______�����ƫ����ƫС��������Ӱ�족����
��3����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����______
A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C������ʵ��ĵ������½ϸ�
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ
E������ȡ����ʱ���Ӽ���
F�����ձ��ĸǰ��м�С��̫��

��1���������ȼƵĹ����ʵ��ijɰܹؼ����жϸ�װ�õĴ�С�ձ���������ֽ���������DZ��¡����ȣ���ֹ����ɢʧ���ʴ�Ϊ�����¡����ȣ���ֹ����ɢʧ��
��2��Ũ������ϡ�����л�ų������������������к��ȵ���ֵ��ƫ�ߣ��ʴ�Ϊ��ƫ��
��3��A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С�����Ե���ʵ�����к��ȵ���ֵƫС����A��ȷ��
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ����ᵼ��һ����������ɢʧ��ʵ�����к��ȵ���ֵƫС����B��ȷ��
C������ʵ������ºͷ�Ӧ�ȵ�����֮���أ���C����
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ�����ڰ�ˮ�������ĵ��������ȵĹ��̣����Ե���ʵ�����к��ȵ���ֵƫС����D��ȷ��
E������ȡ����ʱ���Ӽ�������ʹ��ʵ����ȡ���������Ҫ�������������������Ա�֤��ȫ��Ӧ����ʹ���кͺ��ȵIJⶨ����ƫ�ߣ���E����
F�����ձ��ĸǰ��м�С��̫�ᵼ��һ��������ɢʧ�����Բ����ֵ���ͣ���F��ȷ��
��ѡABDF��
��2��Ũ������ϡ�����л�ų������������������к��ȵ���ֵ��ƫ�ߣ��ʴ�Ϊ��ƫ��
��3��A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С�����Ե���ʵ�����к��ȵ���ֵƫС����A��ȷ��
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ����ᵼ��һ����������ɢʧ��ʵ�����к��ȵ���ֵƫС����B��ȷ��
C������ʵ������ºͷ�Ӧ�ȵ�����֮���أ���C����
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ�����ڰ�ˮ�������ĵ��������ȵĹ��̣����Ե���ʵ�����к��ȵ���ֵƫС����D��ȷ��
E������ȡ����ʱ���Ӽ�������ʹ��ʵ����ȡ���������Ҫ�������������������Ա�֤��ȫ��Ӧ����ʹ���кͺ��ȵIJⶨ����ƫ�ߣ���E����
F�����ձ��ĸǰ��м�С��̫�ᵼ��һ��������ɢʧ�����Բ����ֵ���ͣ���F��ȷ��
��ѡABDF��

��ϰ��ϵ�д�
�����Ŀ
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺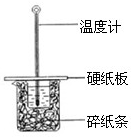 ��1�����ⶨ��20g������������ȼ������ˮ����������2418.0kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ
��1�����ⶨ��20g������������ȼ������ˮ����������2418.0kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ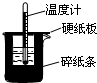 50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺