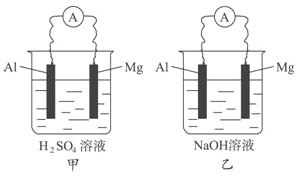
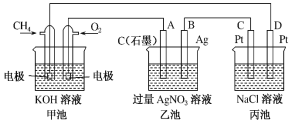
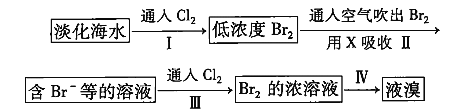
��Ŀ����
����Ŀ�����бȽ�����ȷ����
A. ��ͬŨ�ȵ�������Һ����(NH4)2CO3��Һ����NH4HCO3��Һ����NH4NO3��Һ�� c(NH4+)����>��>��
B. ��ͬpH����Һ����NaClO��Һ����NaHCO3��Һ����CH3COONa��Һ�� c(Na+)����>��>��
C. ͬŨ�ȡ�ͬ�������Һ����CH3COONa��Һ����NH4Cl��Һ����NaNO3��Һ�� pH����>��>��
D. ��ͬŨ�ȵ���Һ������ˮ����NaOH��Һ����Ba(OH)2��Һ c(H+)����>��>��
���𰸡�B
��������
A. ��ͬŨ�ȵ�������Һ������笠����ӵ�ˮ�⣬������ʼŨ�ȼ��������Ӷ���ˮ��̶ȵ�Ӱ�������жϣ�
B. �ȸ�����ͬŨ�ȵ������ж���ˮ��̶ȵ�ǿ��������������˼ά������ͬpH����Һ�У�c(Na+)�Ĵ�С��
C. ������������Һ����������ж���pH��
D. ����Һ�У����ж�c(OH-)�Ĵ�С���ٸ���c(H+) = ![]() ��������
��������
A. ������ˮ�����������Һ�е������c(NH4+)�Ǣڡ�����Һ�е����������������NH4+��HCO3-������ٽ���ˮ�ⷴӦ���ٽ���NH4+��ˮ�⣬��c(NH4+)�Ĵ�С��ϵΪ��>��>�ڣ���A�����
B. ��ͬ�¶ȡ���ͬŨ�ȵ��������ǿ��˳��ΪCH3COOH>H2CO3>HClO����������ˮ����ɣ���ͬ�¶ȡ���ͬŨ�ȵ���������Һ�ļ���ΪNaClO>NaHCO3>CH3COONa������ͬpH����Һ��Na+Ũ�ȴ�С˳��Ϊ��>��>�٣���B����ȷ��
C. CH3COONa��Һˮ���Լ��ԣ�NH4Cl��Һˮ�������ԣ�NaNO3��Һ�����ԣ�����������Һ��pH����>��>�ڣ���C�����
D. ��ˮ��������Һ��NaOH��һԪǿ�Ba(OH)2�Ƕ�Ԫǿ�����ͬŨ�ȵ���Һ��c(OH-)�Ĵ�С��ϵΪ��>��>�٣�c(H+)�Ĵ�С��ϵΪ��>��>�ۣ���D�����
��ѡB��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij���жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5��ֱ��С�ڵ���2.5��m�����������������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش���������
��1����PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
Ũ��/mol��L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
���ݱ��������жϴ�������Ϊ__����ᡱ����ԣ���ʾ����������Ե�c(H+)��c(OH-)=___mol��L-1��
��2��úȼ���ŷŵ������к���SO2��NOx�����γ����꣬��Ⱦ����������NaClO2��Һ�ڼ��������¿ɶ�������������������Ч���dz��á�������ж������������̵����ӷ���ʽ��
��____��ClO2-+��____��NO+��____��OH-=��____��Cl-+��____��NO3-+______
��3��Ϊ����SO2�Ի�������Ⱦ������ú̿ת��Ϊ��������ȼ�ϣ������������д������������е�SO2��
��д����̿��ˮ������Ӧ�Ļ�ѧ����ʽ��__��
���������ʿ�����������������SO2����__������ĸ���ţ���
a.Ca(OH)2 b.Na2CO3 c.CaCl2 d.NaHSO3
��4������β����NOx��CO�����ɼ�ת����
����������ʱ�����¶ȸߣ������л�����NO����ѧ����ʽΪ___��
������ȼ�Ͳ���ȫȼ��ʱ����CO��������β��ϵͳ��װ�ϴ�ת������ʹCO��NO��Ӧת��Ϊ����Ⱦ�����Ե��������壬�仯ѧ��Ӧ����ʽΪ___��