��Ŀ����
17�������£���CH3COOH��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH�������ش�| ��� | c��CH3COOH��/��mol•L-1�� | c ��NaOH��/��mol•L-1�� | �����ҺpH |
| �� | 0.1 | 0.1 | pH=a |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
��2���Ӣ�������������a��7�����������������=������ͬ����
��3���Ӣ�������������c��0.2��
��4���Ӣ�ʵ����������˵��CH3COOH�ĵ���̶ȣ�CH3COONa��ˮ��̶ȣ�CH3COONaˮ������ӷ���ʽ��CH3COO-+H2O?CH3COOH+OH-��
���� ����ΪһԪ���ᣬNaOHΪһԪǿ������£���CH3COOH��NaOH��Һ�������ϣ���ǡ���кͣ�����ǿ�������Σ���Һ�Լ��ԣ���pH=7��˵�������������ͬ���ʵ���Ũ�ȵĴ���������ƵĻ����Һ�����ԣ�˵������ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ��ݴ˷�����
��� �⣺��1��������һԪ���ᣬNaOHΪһԪǿ��䷢���кͷ�Ӧ�����ӷ���ʽΪCH3COOH+OH-=CH3COO-+H2O���ʴ�Ϊ��CH3COOH+OH-=CH3COO-+H2O��
��2�������£���CH3COOH��NaOH��Һ�������ϣ���ǡ���кͣ�����ǿ�������Σ���Һ�Լ��ԣ�pH��7���ʴ�Ϊ������
��3�������£���CH3COOH��NaOH��Һ�������ϣ���ǡ���кͣ�����ǿ�������Σ���Һ�Լ��ԣ���pH=7��˵���������������c��0.2���ʴ�Ϊ������
��4����ͬ���ʵ���Ũ�ȵĴ���������ƵĻ����Һ�����ԣ�˵������ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ����������ˮ������ӷ���ʽΪCH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ������CH3COO-+H2O?CH3COOH+OH-��
���� ���⿼���������ҺpH�ļ��㡢ǿ������ʵĸ���жϣ���ȷ����Ϻ���Һ�е����ʡ�����ˮ�⡢�����Ũ����pH�Ĺ�ϵ���ɽ����Ŀ�Ѷ��еȣ�

| A�� | MnO4-��Mn2+ | B�� | HCO3-��CO2 | C�� | Fe��Fe3+ | D�� | HCl��Cl2 |
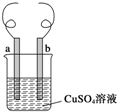 ����˿���缫 a����ͭ˿���缫 b����CuSO4��Һ���Թ���ԭ��ػ���أ���ͼ��ʾ��������˵����ȷ���ǣ�������
����˿���缫 a����ͭ˿���缫 b����CuSO4��Һ���Թ���ԭ��ػ���أ���ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | ����ԭ���ʱ b ����ӦΪ��Cu-2e-�TCu2+ | |
| B�� | ���ɵ���ʱ a ������һ������ | |
| C�� | ���ɵ�ԭ��ػ���ع������ܲ����������� | |
| D�� | ���ɵ���ʱ b ���������ܼ���Ҳ�������� |
| A�� | ��ɫ��Һ�У�Al3+��Cl-��MnO4-��SO42- | |
| B�� | ���д���Fe3+����Һ�У�Na+��Mg2+��NO3-��SCN- | |
| C�� | 0.1 mol•L-1AgNO3��Һ��H+��K+��SO42-��I- | |
| D�� | ʹ��̪���ɫ����Һ��CO32-��Cl-��F-��K+ |
| A�� | ���÷�Һ©����Һʱ��Ӧ��©�����ϵIJ������� | |
| B�� | ����ʵ�鲻һ��ʹ���¶ȼ� | |
| C�� | ��CCl4��ȡ��ˮ�еĵ� | |
| D�� | 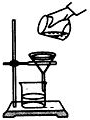 ���ˣ���ͼ��ʱ���ɽ�����Һ���ձ�ֱ�ӵ���©���� ���ˣ���ͼ��ʱ���ɽ�����Һ���ձ�ֱ�ӵ���©���� |
 ��Ӧ���ͣ��Ӿ۷�Ӧ
��Ӧ���ͣ��Ӿ۷�Ӧ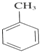 +3HNO3$��_{��}^{Ũ����}$
+3HNO3$��_{��}^{Ũ����}$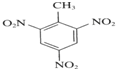 +3H2O��
+3H2O��