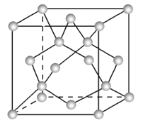
��Ŀ����
����Ŀ���㽭ʦ����ѧ���о��Ŷ��о������һ����и��Ժ�ѡ���Եĸ��ѿ�������![]() ������һ�ɹ�������2020��1��Chem. Mater.�ϡ����и���ɵ�CsPbBr3���������
������һ�ɹ�������2020��1��Chem. Mater.�ϡ����и���ɵ�CsPbBr3���������![]() �̶�������棬���ڿɼ�������CO2��ԭ��
�̶�������棬���ڿɼ�������CO2��ԭ��
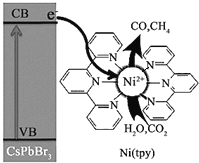
(1)д��Ni��̬ԭ�ӵļ۵����Ų�ͼ���������ʽ��___________________��
(2)C��N��O��Cs��PbԪ�ص�һ�������ɴ�С��˳����____________________��
(3)![]() �Ƕ��������ӣ��������е�ԭ�ӵ��ӻ�����Ϊ_________�������ӽṹ�к���_________������ĸ����
�Ƕ��������ӣ��������е�ԭ�ӵ��ӻ�����Ϊ_________�������ӽṹ�к���_________������ĸ����
a�����Ӽ� b����λ�� C������ d.���
(4)ijЩ��������۵��������±���ʾ��
������ | CO2 | Cs2O | PbO |
�۵�/�� | -56.6 | 490 | 888 |
���ͱ���������֮���۵�����ԭ��______________________________��
(5)��������һ�ְ뵼����ϡ������ṹ�ɿ������ʯ�����ڲ���̼ԭ�ӱ�Nԭ�Ӵ��棬��������ĵ�̼ԭ�ӱ�Gaԭ�Ӵ��档
����ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ__________��
���Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������ꡣ����y��ͶӰ�ľ���������ԭ�ӵķֲ�ͼ��ͼ����2��3��4ԭ�ӵķ������겻���ܵ�����________________��
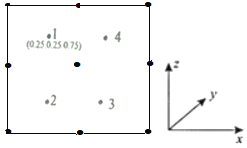
a��(0.75��0.25��0.25) b��(0.25��0.75��0.75)
c��(0.25��0.75��0.25) d��(0.75��0.75��0.75)
�� GaN������N��N��ԭ�Ӻ˼��Ϊa pm��GaNĦ������Ϊ![]() �������ӵ�������ֵΪNA����GaN������ܶ�Ϊ___________
�������ӵ�������ֵΪNA����GaN������ܶ�Ϊ___________![]() ��
��![]() ����
����
���𰸡�![]() N��O��C��Pb��Cs sp2�ӻ� bc Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�� �������� b
N��O��C��Pb��Cs sp2�ӻ� bc Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�� �������� b ![]()
��������
(1)Ni��̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2�����Լ۵����Ų�ͼΪ![]() ����Ϊ��
������![]() ��
��
(2)N��2p������������һ�����ܷ���������ԭ�ӣ��ǽ�����Խǿ����һ������Խ������Խǿ����һ������ԽС������C��N��O��Cs��PbԪ�ص�һ�������ɴ�С��˳����N��O��C��Pb��Cs����Ϊ��N��O��C��Pb��Cs��
(3)��ͼ�п��Կ�������������ÿ����ԭ�Ӷ�������3��ԭ���γɹ��ۼ����۵�����Ϊ3���ӻ�����Ϊsp2�ӻ��������ӽṹ�к�����λ������������ѡbc����Ϊ��sp2�ӻ���bc��
(4)�ӱ������ݿ��Կ��������ߵ��۵��ֵ�ϴɴӾ������ͼ���������������з���������������֮���۵�����ԭ��Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�ߡ���Ϊ��Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�ߣ�
(5)����ͬһ��Gaԭ��������Nԭ�ӣ���4������ľ�����ȣ����ɵĿռ乹��Ϊ�������塣��Ϊ���������壻
�ڸ���1ԭ�ӵķ��������֪2��3��4ԭ�ӵķ�������ֱ�Ϊ(0.25��0.75��0.25)��(0.75��0.25��0.25)��(0.75��0.75��0.75)����ѡb����Ϊ��b��
�� �辧������Ϊx����GaN������N��N��ԭ�Ӻ˼��Ϊ���϶Խ��߳��ȵ�һ�룬��![]() =a pm��x=
=a pm��x=![]() a pm=
a pm=![]() a ��10-10cm���ھ����У�����4����GaN������GaN������ܶ�Ϊ
a ��10-10cm���ھ����У�����4����GaN������GaN������ܶ�Ϊ![]() =
=![]()
![]() ������
������![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ijͬѧ�����������Ũ���ᷴӦ�ĸĽ�װ�ã��������������м��飬ʵ��װ����ͼ��ʾ�����н�������ȷ���ǣ� ��
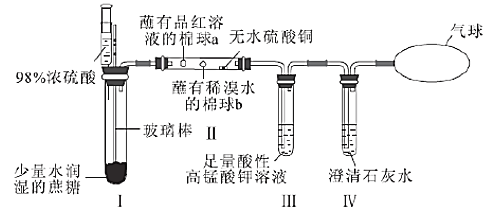
ѡ�� | ���� | ���� |
A. | ����ע��Ũ����ɹ۲쵽�Թ��а�ɫ�����Ϊ��ɫ | ������Ũ�������ˮ�� |
B. | ���й۲쵽����a��b����ɫ | ��������SO2��Ư���� |
C. | ������ˮ����ͭ���� | ˵����Ӧ��������H2O |
D. | ������Һ��ɫ��dz�����г���ʯ��ˮ����� | ˵����CO2���� |
A.AB.BC.CD.D