��Ŀ����
����Ŀ��(1)�õ�ⷨ�ֿ���������Cr2O72-������NO2-�����Է�ˮ[����Cr2O72-ת��ΪCr3+��NO2-ת��Ϊ������]����װ����ͼ��ʾ��
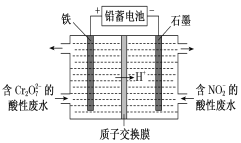
�������缫��Ӧ��________________________��
�����Cr2O72-ת��ΪCr3�������ӷ���ʽ��_________________________________________��
�ڵ�����������2 mol NO2-ʱ���ҳؼ��ٵ�H�������ʵ���Ϊ________ mol��
���������缫����ʯī���������缫��ӦʽΪ��_________________________________��
(2)�ɼ״�(CH3OH)��������NaOH��Һ���ɵ������ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε硣
�ٸõ�ظ����ĵ缫��ӦʽΪ___________________________________________�������缫��Ӧʽ_____________________________________________��
�����Ըõ��Ϊ��Դ����ʯī���缫���200 mL����2mol��L��1HCl��0.5mol��L��1CuSO4�Ļ����Һ���������ռ�����ͬ���(��ͬ������)������ʱ(������Һ����ı仯���缫������ܴ��ڵ��ܽ�����)���������ռ�������������Ϊ_____g���ܹ�ת��_____mol���ӡ�
���𰸡�2NO2-��8H����6e��===N2����4H2O Cr2O72-��6Fe2����14H��===2Cr3����6Fe2����7H2O 2 4OH����4e��===O2����2H2O CH3OH��6e����8OH��===CO32-��6H2O O2+4e����2H2O===4OH�� 3.2 g 0.8mol
��������
(1) ������������ԭ��Ӧ���������Ӿ��л�ԭ�ԣ��ܱ��ظ��������Ϊ�����ӣ��ݴ���д��
�ڸ��������缫��Ӧʽ2NO2-��8H����6e��===N2����4H2O��������������2 mol NO2-ʱ��ͬʱ����8molH+��ͬʱ���ݵ�ʧ�����غ㣬ת��6mol���ӣ�����6molH+����ؽ����ҳأ��Դ˷�����
���������缫����ʯī����ʯīΪ���Ե缫������������Һ�е�����������ʧ���ӷ���������Ӧ��
(2)�ټ״�ȼ�ϵ���м״��ڸ���ʧ���ӷ���������Ӧ�������������õ��ӷ�����ԭ��Ӧ���ڼ���Һ������̼���Σ�
�ڿ�ʼ�Σ������缫��ӦΪ��Cu2++2e-=Cu�������缫��ӦΪ��2Cl��2e-=Cl2�������һ��ʱ��������ռ�����ͬ���(��ͬ����)�����壬������������������������������Ӧ��2H++2e-=H2����������������4OH��4e-=2H2O+O2����������������Ϊ0.2mol����������Ϊxmol��������Ϊ(x+0.2)mol�����ݵ���ת���غ��з��̼�����
(1) �ٵ��ص�����������ԭ��Ӧ����Һ�е�NO2-�õ��ӣ��缫��ӦʽΪ��2NO2-��8H����6e��===N2����4H2O��Cr2O72-��Fe2+����ΪFe3+��Cr2O72-����ԭΪCr3+������Ԫ���غ�ͬʱ����H2O���������ӷ���ʽ��Cr2O72-��6Fe2����14H��===2Cr3����6Fe2����7H2O��
��ˣ�������ȷ���ǣ�2NO2-��8H����6e��===N2����4H2O��Cr2O72-��6Fe2����14H��===2Cr3����6Fe2����7H2O��
�ڸ��������缫��Ӧʽ2NO2-��8H����6e��===N2����4H2O��������������2 mol NO2-ʱ��ͬʱ����8molH+��ͬʱ���ݵ�ʧ�����غ㣬ת��6mol���ӣ�����6molH+����ؽ����ҳأ������ҳؼ��ٵ�H�������ʵ���Ϊ2mol��
��ˣ�������ȷ���ǣ�2��
���������缫����ʯī����ʯīΪ���Ե缫������������Һ�е�����������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��4OH����4e��===O2����2H2O��
��ˣ�������ȷ���ǣ�4OH����4e��===O2����2H2O��
(2)�ٸ�ȼ�ϵ���У���ص�����ͨ������������ͨ��ȼ�ϼ״��������������������Һ������O2�õ������������������ӣ�O2+4e����2H2O===4OH���������缫��Ӧ��CH3OH��6e����8OH��===CO32-��6H2O��
��ˣ�������ȷ���ǣ�CH3OH��6e����8OH��===CO32-��6H2O��O2+4e����2H2O===4OH����
����Һ��n(Cu2+)=0.2L![]() 0.5mol/L=0.1mol��n(H+)=0.2L
0.5mol/L=0.1mol��n(H+)=0.2L![]() 2mol/L=0.4mol��n(Cl-)=0.2L
2mol/L=0.4mol��n(Cl-)=0.2L![]() 2mol/L=0.4mol��
2mol/L=0.4mol��
��ʼ�Σ������缫��ӦΪ��Cu2++2e-=Cu�������缫��ӦΪ��2Cl��2e-=Cl2�������һ��ʱ��������ռ�����ͬ���(��ͬ��)�����壬������������������������������Ӧ��2H++2e-=H2����������������4OH��4e-=2H2O+O2����������������Ϊ0.2mol����������Ϊxmol��������Ϊ(x+0.2)mol�����ݵ���ת���غ㣬��0.1mol![]() 2+(x+0.2)mol
2+(x+0.2)mol![]() 2=0.2mol
2=0.2mol ![]() 2+xmol
2+xmol![]() 4������ó�x=0.1��
4������ó�x=0.1��
���ռ���������Ϊ0.1mol������������Ϊ0.1mol![]() 32g/mol=3.2g��
32g/mol=3.2g��
�ܹ�ת�Ƶ���Ϊ0.2mol![]() 2+0.1mol
2+0.1mol![]() 4=0.8mol��
4=0.8mol��
��ˣ�������ȷ���ǣ�3.2 g ��0.8mol��