��Ŀ����
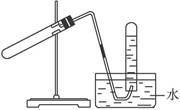
ʵ��������ϩʱ��������������ʹBr2�����Ȼ�̼��Һ��ɫ���ס���ͬѧ������ʵ����֤�����������Ѽ��飬���ּг�װ���ԣ���
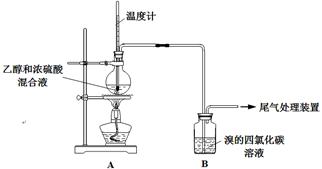
ʵ�����������
��1����ƿ�ڲ�����ϩ�Ļ�ѧ����ʽ��___ __ ��
��2����Һ��������ڡ���˵��Ũ������� �ԡ�
��3������ʹB����Һ��ɫ�����ʣ�����Ϊ��C2H4������Ϊ�����ų�SO2�����á�
�� ���ݼĹ۵㣬ʹB����Һ��ɫ��Ӧ�Ļ�ѧ����ʽ�� ��
�� �Ҹ����������Ϊ������SO2����B����SO2��Ӧʹ��Һ��ɫ�������� ��
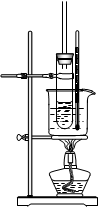
�� Ϊ֤ʵ���Թ۵㣬�ס�������ʵ�飬������������£�
a. ���ݼ���ƣ�ϴ��ƿ��ʢ�ŵ��Լ���___ ___��
b. �����ҵ���ƣ�C��ʢ�ŵ��Լ���___ __��
c. ��˵��ȷʵ��SO2ʹE����Һ��ɫ��ʵ����_ ___��
d. ��Ϊ��һ����֤��۵㣬ȡ����D����Һ�����뼸��BaCl2��Һ��������������ɫ������dz����ɫ��ʧ��������Ӧ�����ӷ���ʽ��____ _��
��4������ʵ��õ��Ľ����� ��

ʵ�����������
| �� �� | �� �� |
| ��ȼ�ƾ��ƣ� ������170�� | ��A����ƿ��Һ�彥����� ��B����������ð������Һ����ɫ |
| ���� | |
| ʵ����ϣ� ��ϴ��ƿ | ��A����ƿ�ڸ���������ɫ����״��д̼�����ζ�ݳ� |
��2����Һ��������ڡ���˵��Ũ������� �ԡ�
��3������ʹB����Һ��ɫ�����ʣ�����Ϊ��C2H4������Ϊ�����ų�SO2�����á�
�� ���ݼĹ۵㣬ʹB����Һ��ɫ��Ӧ�Ļ�ѧ����ʽ�� ��
�� �Ҹ����������Ϊ������SO2����B����SO2��Ӧʹ��Һ��ɫ�������� ��
�� Ϊ֤ʵ���Թ۵㣬�ס�������ʵ�飬������������£�
| | �� �� | �� �� |
| �� | ��A��B������һ��װ��ij���Լ���ϴ��ƿ | Br2��CCl4��Һ��ɫ |
| �� | ��A���ӵ�װ�����£� | D����Һ�ɺ���ɫ��Ϊdz����ɫʱ��E����Һ��ɫ |
b. �����ҵ���ƣ�C��ʢ�ŵ��Լ���___ __��
c. ��˵��ȷʵ��SO2ʹE����Һ��ɫ��ʵ����_ ___��
d. ��Ϊ��һ����֤��۵㣬ȡ����D����Һ�����뼸��BaCl2��Һ��������������ɫ������dz����ɫ��ʧ��������Ӧ�����ӷ���ʽ��____ _��
��4������ʵ��õ��Ľ����� ��
��1�� ��2�֣�
��2�֣�
��2����ˮ�ԣ�1�֣�
��3���� CH2=CH2 + Br2�� CH2Br��CH2Br��2�֣� �� H2O ��Br2��2�֣�
�� a NaOH ��Һ��2�֣� b Ũ���ᣨ2�֣�
c �����Ѿ���ɫ��Ʒ����Һ������ɫ�ָ���֤����SO2ʹƷ����Һ��ɫ������Br2 ��2�֣�
d SO2 + 2H2O +Br2 ="=" 4H+ +2Br- + SO42- ��SO42- + Ba2+ ="=" BaSO4�� ��2�֣�
�� SO2 + 2H2O +Br2 + Ba2+ ="=" 4H+ + 2Br- + BaSO4��
��4����ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ�������SO2����ʹBr2�����Ȼ�̼��Һ��ɫ
 ��2�֣�
��2�֣���2����ˮ�ԣ�1�֣�
��3���� CH2=CH2 + Br2�� CH2Br��CH2Br��2�֣� �� H2O ��Br2��2�֣�
�� a NaOH ��Һ��2�֣� b Ũ���ᣨ2�֣�
c �����Ѿ���ɫ��Ʒ����Һ������ɫ�ָ���֤����SO2ʹƷ����Һ��ɫ������Br2 ��2�֣�
d SO2 + 2H2O +Br2 ="=" 4H+ +2Br- + SO42- ��SO42- + Ba2+ ="=" BaSO4�� ��2�֣�
�� SO2 + 2H2O +Br2 + Ba2+ ="=" 4H+ + 2Br- + BaSO4��
��4����ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ�������SO2����ʹBr2�����Ȼ�̼��Һ��ɫ
�����������1��ע����ϩ����ȡ�¶���170�棬140�������£��Ҵ�����ȡ����Ӧ���������ѣ���2��Ũ�������ˮ�ԣ�ʹ����Һ��ڣ���3������ϩ���巢���ӳɷ�Ӧ��������ΪBr2�����Ȼ�̼��Һ��ɫ������Ϊ�����˷�ӦSO2 + 2H2O +Br2 ="=" 4H+ +2Br- + SO42- ������B����SO2��Ӧʹ��Һ��ɫ��������H2O ��Br2����NaOH ��Һ�ܳ�ȥ��������������ͨ��Ũ���ᣬ��ȥˮ�֣���SO2��H2O ��Br2���߲��ᷢ����Ӧ����Br2�����Ȼ�̼��Һ��ɫֻ������ϩ����ļӳɷ�Ӧ������������ʹƷ����Һ��ɫ�������Ⱥ�ɫ�ָֻ���
�������Ի�ѧʵ��Ŀ���������ĸ߿��ص㣬�����ڱ�����Ӧע��Ի�ѧʵ�顢��ѧ���������֪ʶ�Ļ��ۡ�

��ϰ��ϵ�д�
�����Ŀ