��Ŀ����
����Ŀ��(1)���������õ������£�NH4+����������Ӧ��������NO3-��������Ӧ�������仯ʾ��ͼ���£�

1 mol NH4+(aq)ȫ��������NO3-(aq)���Ȼ�ѧ����ʽ��___________________��
(2)��ҵ�ϳ���CO��H2�ϳɼ״�����Ӧ����ʽΪ��CO(g) ��2H2(g)![]() CH3OH (g)����H����T1ʱ�����Ϊ2 L�ĺ��������г������ʵ���֮��Ϊ3 mol��H2��CO���ﵽƽ��ʱCH3OH���������(V %)��n(H2)/n(CO)�Ĺ�ϵ��ͼ1��ʾ��
CH3OH (g)����H����T1ʱ�����Ϊ2 L�ĺ��������г������ʵ���֮��Ϊ3 mol��H2��CO���ﵽƽ��ʱCH3OH���������(V %)��n(H2)/n(CO)�Ĺ�ϵ��ͼ1��ʾ��

�ٵ���ʼn(H2)/n(CO)��2������5 min�ﵽƽ�⣬��ʱ������ѹǿ�dz�ʼѹǿ��0.7������0��5 min��ƽ����Ӧ����v(H2)��________������ʱ���������м���0.15 mol CO (g)��0.05 mol CH3OH(g)������ƽ��ʱH2��ת���ʽ�________(��������������С������������)��
�ڵ���ʼn(H2)/n(CO)��3.5ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�____��(����D����E������F��)��
����ͼ2��֪�÷�Ӧ����H________0(����>����<����������)��������______________����ѹǿΪp2ʱ����y�㣺v��________v��(����>����<����������)��
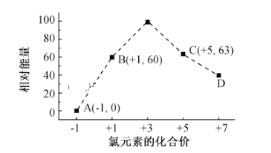
���𰸡�NH4+��aq��+2O2��g���T2H+��aq��+H2O��l��+NO3-��aq������H=-346 kJ/mol 0.09mol/(L.min) ���� F > �¶����ߣ�һ����̼��ת��������ƽ��������� >
��������
��1�����ͼ����ݸ�˹���������㷴Ӧ���ʱ䣻
��2������CO�����������ʵ���n(H2)/n(CO)��2
���Լ���CO���������Ե����ʵ�������������ʽ����ƽ��ʱ��������ʵ�������������ʵ����仯��������v=c��t������v��H2����
�ȼ�����¶���ƽ�ⳣ��K���ټ���Ũ����Qc����ƽ�ⳣ��K����жϷ�Ӧ���з���������ȷ������ת���ʱ仯��
�ڻ�ϱ������ڻ�ѧ������֮��ʱ��ƽ��ʱ������ĺ������
��1����һ�����Ȼ�ѧ����ʽΪNH4+��aq��+1.5O2��g���TNO2-��aq��+2H+��aq��+H2O��l������H=-273KJ/mol��
�ڶ������Ȼ�ѧ����ʽΪ��NO2-��aq��+0.5O2��g���TNO3-��aq������H=-73KJ/mol��
���ݸ�˹������NH4+��aq��+2O2��g���T2H+��aq��+H2O��l��+NO3-��aq������H=-346 kJ/mol��
�ʴ�Ϊ��NH4+��aq��+2O2��g���T2H+��aq��+H2O��l��+NO3-��aq������H=-346 kJ/mol��
(2)��H2��CO�ܹ�Ϊ3mol,����ʼn(H2)/n(CO)=2,��֪H2Ϊ2mol��COΪ1mol��5min�ﵽƽ��ʱ��ʱ������ѹǿ�dz�ʼѹǿ��0.7�����������ĵ�һ����̼Ϊxmol��
CO(g) ��2H2(g)![]() CH3OH (g)
CH3OH (g)
��ʼ(mol): 1 2 0
�仯(mol): x 2x x
ƽ��(mol): 1-x 2-2x x
(1-x+2-2x+ x)��3=0.7 ��ã�x=0.45
�������ݻ�Ϊ2L,��v(H2)=0.9mol��2L��5min=0.09mol/(L.min)��
���¶���ƽ�ⳣ��K=0.225��(0.275��(0.55)2),��ʱ����������0.15 mol CO (g)��0.05 mol CH3OH(g)����ʱŨ����Qc=0.25��(0.35��(0.55)2)<K=0.225��(0.275��(0.55)2)����Ӧ������Ӧ���У�����ƽ��ʱH2��ת���ʽ�����
�ʴ�Ϊ��0.09mol/(L.min)������
�ڻ�ϱ������ڻ�ѧ������֮��ʱ,ƽ��ʱ������ĺ������,�ʵ�n(H2)/n(CO)��3.5ʱ,�ﵽƽ��״̬��,CH3OH���������С��C�㣬��ѡF��
�ʴ�Ϊ��F��
����ͼ2��֪���ڵ�ѹ���������¶ȵ����ߣ�һ����̼��ת���������������¶ȵ����߷�Ӧ������У�������Ӧ�����ȷ�Ӧ����H>0����ѹǿΪp2ʱ����y��δ����ƽ��״̬����ѹǿ���¶���Ҫ����ƽ��״̬��һ����̼��ת����Ҫ����������Ӧ������У���v��>v��
�ʴ�Ϊ��>���¶����ߣ�һ����̼��ת��������ƽ��������У�>��

����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������з�Ӧ��2N2O5(g) ![]() 4NO2(g) + O2(g) ��H ��+Q kJ/mol (Q>0)��ij�¶��£���2L���ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
4NO2(g) + O2(g) ��H ��+Q kJ/mol (Q>0)��ij�¶��£���2L���ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
ʱ��/s | 0 | 500 | 1000 | 1500 |
c(N2O5)/mol/L | 5.0 | 3.5 | 2.5 | 2.5 |
����˵����ȷ����
A. 500s��N2O5�ֽ�����Ϊ6��10-3mol/(L��s)
B. ���¶��µ�ƽ�ⳣ��K ��125
C. ��Ӧ��ƽ��ʱ�����յ�����Ϊ5Q kJ
D. �����������䣬����ʼʱc(N2O5)��10mol/L�����ƽ���c(N2O5)��5mol/L
����Ŀ������˵������ȷ���ǣ� ��
A.HF��HCl��![]() ��
��![]() ���ȶ���������ǿ
���ȶ���������ǿ
B.��Mg��Si��N��F��˳��ԭ�Ӱ뾶��С���
C.ij����Ԫ�صĵ�����![]() �������±���ʾ
�������±���ʾ![]() ��λ��
��λ��![]() �����Ʋ��Ԫ��λ��Ԫ�����ڱ��ڢ�A��
�����Ʋ��Ԫ��λ��Ԫ�����ڱ��ڢ�A��
I | I | I | I | I | I | I |
578 |
|
|
|
|
|
|
D.�ڢ�P��S����![]() ��Ca����
��Ca����![]() ��Si����Ԫ���У�ÿ���е�һ�����ܽϴ��Ԫ�ص�ԭ������֮��Ϊ41
��Si����Ԫ���У�ÿ���е�һ�����ܽϴ��Ԫ�ص�ԭ������֮��Ϊ41