��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣��ش��������⡣

��1���������ڱ��е�λ����_______________����ԭ�ӽṹʾ��ͼΪ______________��
��2���ݢޢߵļ����Ӱ뾶��С����______�������ӷ��ţ���ԭ�Ӱ뾶��С����_______���ѧʽ�����ݢޢ�����Ԫ�ص���̬�⻯�����ȶ�����______���û�ѧʽ��ʾ����
��3����Ӧ����̬�⻯��е����ͬ����Ԫ�ض�Ӧ����̬�⻯�ԭ����_____________��
��4���٢���Ԫ�ؿ����γ�ԭ�Ӹ�����1�U1��2�U1�Ļ�����X��Y������X�ĵ���ʽΪ___________���õ���ʽ��ʾY���γɹ���____________________________________��
��5���ɢ���������Ԫ����ɵĻ�����֮һZ���������г������л�������������������ζ��д��Z��ߵķ�Ӧ����ʽ��___________________________________��
���𰸡� �������ڢ�A�� ![]() Na+ F HF HF���Ӽ������������ǿ�ڷ��»���
Na+ F HF HF���Ӽ������������ǿ�ڷ��»��� ![]()
![]() 2CH3CH2OH+2Na��2CH3CH2ONa+H2��
2CH3CH2OH+2Na��2CH3CH2ONa+H2��
������������Ԫ�������ڱ��е����λ�ÿ�֪������ֱ���H��Be��C��N��O��F��Na��Al��Cl��Ar��
��1��Cl����������17�������ڱ��е�λ���ǵ������ڢ�A�壬��ԭ�ӽṹʾ��ͼΪ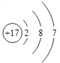 ����2�����ӵĺ�����Ӳ���Խ�࣬���Ӱ뾶Խ��������Ų���ͬʱ���Ӱ뾶��ԭ���������������С����ݢޢߵļ����Ӱ뾶��С����Na+��ͬ������������ԭ�Ӱ뾶��С��ͬ������ϵ���ԭ�Ӱ뾶����������ԭ�Ӱ뾶��С����F���ݢޢ�����Ԫ����F�ķǽ�������ǿ������̬�⻯�����ȶ�����HF����3������HF���Ӽ������������ǿ�ڷ��»���������F��Ӧ����̬�⻯��е����ͬ����Ԫ�ض�Ӧ����̬�⻯���4���٢���Ԫ�ؿ����γ�ԭ�Ӹ�����1�U1��2�U1�Ļ�����X��Y�ֱ���˫��ˮ��ˮ������˫��ˮ�ĵ���ʽΪ
����2�����ӵĺ�����Ӳ���Խ�࣬���Ӱ뾶Խ��������Ų���ͬʱ���Ӱ뾶��ԭ���������������С����ݢޢߵļ����Ӱ뾶��С����Na+��ͬ������������ԭ�Ӱ뾶��С��ͬ������ϵ���ԭ�Ӱ뾶����������ԭ�Ӱ뾶��С����F���ݢޢ�����Ԫ����F�ķǽ�������ǿ������̬�⻯�����ȶ�����HF����3������HF���Ӽ������������ǿ�ڷ��»���������F��Ӧ����̬�⻯��е����ͬ����Ԫ�ض�Ӧ����̬�⻯���4���٢���Ԫ�ؿ����γ�ԭ�Ӹ�����1�U1��2�U1�Ļ�����X��Y�ֱ���˫��ˮ��ˮ������˫��ˮ�ĵ���ʽΪ![]() ��ˮ���γɹ��̿ɱ�ʾΪ
��ˮ���γɹ��̿ɱ�ʾΪ![]() ����5���ɢ٢ۢ�����Ԫ����ɵĻ�����֮һZ�������г������л�������������������ζ��Z���Ҵ����Ҵ����Ʒ�Ӧ�ķ���ʽΪ2CH3CH2OH+2Na��2CH3CH2ONa+H2����
����5���ɢ٢ۢ�����Ԫ����ɵĻ�����֮һZ�������г������л�������������������ζ��Z���Ҵ����Ҵ����Ʒ�Ӧ�ķ���ʽΪ2CH3CH2OH+2Na��2CH3CH2ONa+H2����

 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�����Ŀ������˼ά�ǻ�ѧ�����г��õ�һ��˼ά�����������йط�Ӧ����ʽ��������ȷ����
�� ֪ | �� �� | |
A | ������Ͷ��ϡ������ Fe+H2SO4(ϡ)= FeSO4+H2�� | ������Ͷ��ϡ������ Fe+2HNO3(ϡ)= Fe(NO3)2+H2�� |
B |
|
|
C | ����ϩͨ����ˮ�� CH2��CH2+Br2��CH2BrCH2Br | ����ϩͨ����ˮ�� CH3CH��CH2+Br2��CH3CHBrCH2Br |
D | ��ϩ���ʵ��������ƾ���ϩ nCH2��CH2 | ��ϩ���ʵ��������ƾ۱�ϩ nCH3CH��CH2 |
A. A B. B C. C D. D
����Ŀ�����������ѳ�Ϊ����Ĺ�ʶ����������������Ӧ�Ļ�������û�й�ϵ����
A | B | C | D |
�������� | ������̼ | ±������ | �������� |
��ɫ��Ⱦ | ����ЧӦ | ���������� | ���� |
A. A B. B C. C D. D



