��Ŀ����
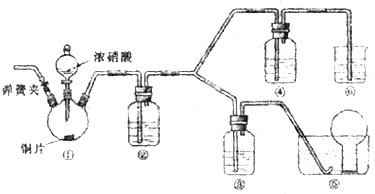
����ͼװ�ý���ʵ�飬��֪C1��C2Ϊʯī����

�ش���������:
��1���ж�װ�õ����ƣ�A��Ϊ�ߣߣߣߣߣߣߣ�B��Ϊ�ߣߣߣߣߣߣߣߣ�
��2��п��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ͭ��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1Ϊ�ߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1����������ʵ������Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3����C1������224mL���壨��״���£�ʱ��п�������ߣߣߣߣ�����ӡ��������䡱���١����ˣߣߣߣߣ�g��CuSO4��Һ�������ߣߣߣߣ�����ӡ��������䡱���١����ߣߣߣߣ�g��
���𰸡�
��1������ ԭ���
��2������Zn-2e-=Zn2+������Cu2++2e-= Cu��������4OH--4e-=2H2O+O2�����������ݣ�ͬʱ��Һ��ɫ���
��3�����١�1.3����С��1.6
��������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
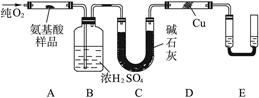
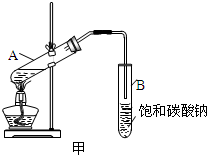 ij����С��Լ�����������µ�ʵ�飬����֤�京��ȩ�����������仯ѧ���ʣ���������������Ӧ��
ij����С��Լ�����������µ�ʵ�飬����֤�京��ȩ�����������仯ѧ���ʣ���������������Ӧ��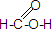 ��CH3CH2-18OH����������Ӧ�Ļ�ѧ����ʽ
��CH3CH2-18OH����������Ӧ�Ļ�ѧ����ʽ