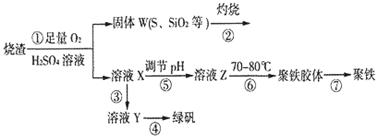
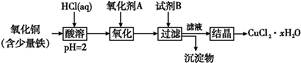
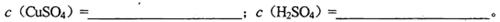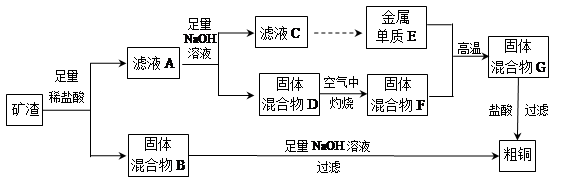��Ŀ����
��֪Fe2O3��H2��Ӧ�����¶Ȳ�ͬ��������Fe3O4���ɡ�ij��ѧ��ȤС������H2��ԭFe2O3��ʵ���У��ô����������ɵĺ�ɫ��ĩX��Ϊ̽��X����ɣ����ǽ���������ʵ�飺
(1)��ͬѧ��Ϊ��ɫ��ĩX�ܱ��������������X����������ͬѧ��ͬ�����Ľ��ۣ�ԭ����___________________________��
(2)��ͬѧ�Ƚ�������ɫ��ĩX����װ����������ͭ��Һ���ձ��У������岿���ܽ⣬�м�������ɫ�������������ˣ�Ȼ���������м������ᣬ�ٵμӼ���KSCN��Һ����Һ���ֺ�ɫ��ͨ�������������ͬѧ�ó�X�������Fe��Fe3O4��
�ٵμ�KSCN��Һ��Ŀ����_____________________��
��������ĩXֱ�Ӽ��������У��ټ�KSCN��Һ����Һ�����ֺ�ɫ�����ֺ�ɫ��ԭ����(�����ӷ���ʽ��ʾ) _____________��
(3)��ͬѧ��ʵ�鷽����

�ٲ���Z��__________________��
��ͨ���������ݣ��ó�2.88 g��ɫ��ĩX�и��ɷֵ����ʵ���Ϊ____________________________________��
(1)��ͬѧ��Ϊ��ɫ��ĩX�ܱ��������������X����������ͬѧ��ͬ�����Ľ��ۣ�ԭ����___________________________��
(2)��ͬѧ�Ƚ�������ɫ��ĩX����װ����������ͭ��Һ���ձ��У������岿���ܽ⣬�м�������ɫ�������������ˣ�Ȼ���������м������ᣬ�ٵμӼ���KSCN��Һ����Һ���ֺ�ɫ��ͨ�������������ͬѧ�ó�X�������Fe��Fe3O4��
�ٵμ�KSCN��Һ��Ŀ����_____________________��
��������ĩXֱ�Ӽ��������У��ټ�KSCN��Һ����Һ�����ֺ�ɫ�����ֺ�ɫ��ԭ����(�����ӷ���ʽ��ʾ) _____________��
(3)��ͬѧ��ʵ�鷽����

�ٲ���Z��__________________��
��ͨ���������ݣ��ó�2.88 g��ɫ��ĩX�и��ɷֵ����ʵ���Ϊ____________________________________��
(1)Fe3O4Ҳ�ܱ������������ʲ����ɴ�ȷ��X����������
(2)�ټ����Ƿ����Fe3����ȷ��Fe3O4�Ĵ���
��Fe��2Fe3��=3Fe2��
(3)�ٹ��ˡ�ϴ��
��n(Fe3O4)��0.01 mol��n(Fe)��0.01 mol
(2)�ټ����Ƿ����Fe3����ȷ��Fe3O4�Ĵ���
��Fe��2Fe3��=3Fe2��
(3)�ٹ��ˡ�ϴ��
��n(Fe3O4)��0.01 mol��n(Fe)��0.01 mol
��ͨ������Z�õ����������Ըò����ǹ��ˡ�ϴ�ӣ��������⣬n(Fe)��56��n(Fe3O4)��232��2.88����n(Fe)��3n(Fe3O4)�ݡ�160/2��3.2����ã�n(Fe)��0.01 mol��n(Fe3O4)��0.01 mol

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
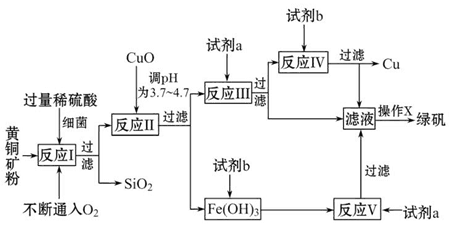
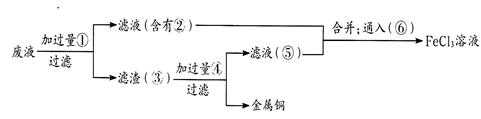
 CuSO4��1.0 mol
CuSO4��1.0 mol