��Ŀ����
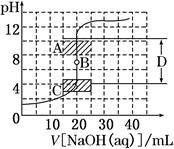
��a mL������Һ�еμ�0.01 mol��L��1������������Һ���ζ�������ͼ��ʾ��

(1)������ҺŨ��________(����ڡ���С�ڡ����ڡ�)0.01 mol��L��1��������________��
(2)b�㣬c(Na��)________c(CH3COO��)(�>����<������)
(3)������������������Һǡ����ȫ�к�ʱ�������϶�Ӧ�ĵ�QӦ��____��
A��2��a֮�� B��a��b֮��
C��b��c֮�� D��a��c֮��
(4)���й�ϵʽһ����ȷ����________��
A��a�㣬c(H��)>c(OH��)>c(CH3COO��)>c(Na��)
B��a�㣬c(Na��)>c(CH3COO��)>c(H��)>c(OH��)
C��c�㣬c(Na��)>c(CH3COO��)>c(OH��)>c(H��)
D��c�㣬c(Na��)��c(H��)��c(CH3COO��)��c(OH��)

(1)������ҺŨ��________(����ڡ���С�ڡ����ڡ�)0.01 mol��L��1��������________��
(2)b�㣬c(Na��)________c(CH3COO��)(�>����<������)
(3)������������������Һǡ����ȫ�к�ʱ�������϶�Ӧ�ĵ�QӦ��____��
A��2��a֮�� B��a��b֮��
C��b��c֮�� D��a��c֮��
(4)���й�ϵʽһ����ȷ����________��
A��a�㣬c(H��)>c(OH��)>c(CH3COO��)>c(Na��)
B��a�㣬c(Na��)>c(CH3COO��)>c(H��)>c(OH��)
C��c�㣬c(Na��)>c(CH3COO��)>c(OH��)>c(H��)
D��c�㣬c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
(1)���ڣ�CH3COOH��������ʣ�ֻ���ֵ���
(2)����(3)C��(4)D
(2)����(3)C��(4)D
(1)��ʼʱ��������Һ��pH��2��c(H��)��0.01 mol��L��1�����ڴ�����������ʣ�������Һ�д���δ�����CH3COOH����c(CH3COOH)>0.01 mol��L��1��(2)b�㣬pH��7��ʾc(H��)��c(OH��)���ɵ���غ�ʽc(Na��)��c(H��)��c(OH��)��c(CH3COO��)�ó���c(Na��)��c(CH3COO��)��(3)CH3COOH��NaOH=CH3COONa��H2O������������������ǡ����ȫ�к�ʱ����Һ�ʼ��ԣ�pH>7�������϶�Ӧ��Q��Ӧ��b��c֮�䡣(4)a�㣬��Һ�������Ǵ���ʹ����ƣ���Һ�����ԣ����������ᣬ����Ũ�ȴ�С��ϵΪ��c(CH3COO��)>c(H��)>c(OH��)��������Һ�����ԡ����Ի���ԣ����ж�ֻ����Na����H����CH3COO����OH��������һ���е���غ�ʽ��Dѡ����ȷ��

��ϰ��ϵ�д�
 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
�����Ŀ