��Ŀ����
��12�֣��ס��ҡ����������ֶ�����Ԫ�ؿ���������п�ͼ�г�Br2��L��������ʣ���ԭ���������������ҿ��γɳ���Һ̬������K������A�к��б�Ԫ�ص���һ�������ӣ�����Ӳ�ṹ����ԭ����ͬ����Ԫ��ԭ�ӵ�����������������Ӳ�����2������һ�������£����и����ʿɷ�����ͼ��ʾ�ı仯����Ӧ�����ɵ�ˮû��д������
�Իش�
��1����Ԫ�ص�����Ϊ ����Ԫ�������ڱ���λ�� ��Ԫ�ص������ӽṹʾ��ͼΪ ��
��2��A�ĵ���ʽΪ ��������ѧ������Ϊ ��
��3���ҡ���������Ԫ�ص����Ӱ뾶�ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ�� ��
��4����Ӧ��I���Ļ�ѧ����ʽΪ ��
��5����Ӧ��II�������ӷ���ʽΪ ��
(1)��(1��)���������ڢ�A��(1��)�� 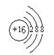 (1��)
(1��)
��2��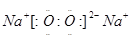 (1��)�����Ӽ����ۼ�(2��)
(1��)�����Ӽ����ۼ�(2��)
��3��S2->O2->Na+(2��)
��4�� 2Na2O2+2H2O=4NaOH+O2����2�֣�
��5��SO2��Br2��2H2O��4H+ + SO42--�� 2Br-��2�֣�
�����רDH���ҨDO�����DNa�����DS A�DNa2O2��B�DNa2SO3��C�DNaOH��D�DO2��E�DH2SO4��F�DSO2��G�DNa2SO4��H�DSO3��K�DH2O��L�DHCl��
(1)��(1��)���������ڢ�A��(1��)��  (1��)
(1��)
��2��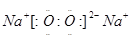 (1��)�����Ӽ����ۼ�(2��)
(1��)�����Ӽ����ۼ�(2��)
��3��S2->O2->Na+(2��)
��4�� 2Na2O2+2H2O=4NaOH+O2����2�֣�
��5��SO2��Br2��2H2O��4H+ + SO42--�� 2Br-��2�֣�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� ��֪�ס��ҡ����������ֶ�����Ԫ�������ڱ��е�λ����ͼ��ʾ��������˵���в���ȷ���ǣ�������
��֪�ס��ҡ����������ֶ�����Ԫ�������ڱ��е�λ����ͼ��ʾ��������˵���в���ȷ���ǣ�������


