��Ŀ����
��ѧ�ܵ�ת������ʵ�����еõ��˹㷺�����á��ش��������⣺
����(1)��25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ�յ��Ȼ�ѧ����ʽ�� ��
(2)2Zn��s��+O2��g��=2ZnO��s�� ��H1=" ��702" kJ/mol
2Hg��l��+O2��g��=2HgO��s�� ��H2=" ��182" kJ/mol
�ɴ˿�֪ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3= ��
��3��20����30�����Eyring��Pelzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ������������̬����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� 
������ͼΪ������ļ����������أ�
��ش�
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���
A�������������������� ���缫��ӦΪ����������������������
B�������������������� ���缫��ӦΪ����������������������
�������ҺΪ������������������
��2���ҳ���������������̪��Һ����ʼһ��ʱ���Fe������������������ɫ��
��3�����ײ���������12��8g�����Ҳ������ų������ڱ�״���µ����
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ͬʱ���Ҳ�ʣ��Һ��Ϊ400mL�������õ���Һ�����ʵ���Ũ��Ϊ
__ ������ _____��
����1��CH4��g��+2O2��g��==== CO2��g��+ 2H2O��g�� ��H =" ��880" kJ/mol
��2��+260 kJ/mol
��3��NO2��g��+CO��g��="===" CO2��g��+NO��g������H =" ��234" kJ/mol
����1��������ͭ��Cu2++2e-=Cu��������ͭ��Cu-2e-=Cu2+ CuSO4��Һ
��2����
��3��4.48 L
��4��1mol/L
����

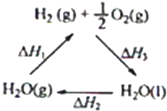 ij��ѧ�����ö������棨CeO2����̫���������½�H2O��CO2ת���H2��CO����������£�
ij��ѧ�����ö������棨CeO2����̫���������½�H2O��CO2ת���H2��CO����������£�mCeO2
| ̫���� |
| �� |
��m-x��CeO2?xCe+xH2O+xCO2
| 900�� |
| �� |
����˵������ȷ���ǣ�������
| A���ù�����CeO2û������ |
| B���ù���ʵ����̫������ѧ�ܵ�ת�� |
| C��ͼ�С�H1=��H2+��H3 |
| D����CO��O2���ɵļ���ȼ�ϵ�صĸ�����ӦʽΪCO+4OH--2e-=CO32-+2H2O |


