��Ŀ����
�й�����ֵ�������ʾ��2013��ȫ��ƽ����������Ϊ52����֮��γ���������Ҫ�ɷ�Ϊ�������������ŷŵķ���������β�����ﳾ�ȡ�
��1����CH4������������β���е����������Ⱦ��
��֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(l) ��H����955 kJ/mol
2NO2(g)��N2O4(g) ��H����56.9 kJ/mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ ��
��2����֪��CO(g)��H2O(g)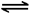 CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g)
CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g)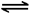 CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ��
CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ��
��3����ʽ��������������������Ҫ����������
����Al2(SO4)3��Һ��Ͷ���ĩ״ʯ��ʯ�����ɼ�ʽ������[Al2(SO4)3��Al2O3]��Һ��
�ڼ�ʽ����������SO2��Al2(SO4)3��Al2O3+3SO2��Al2(SO4)3��Al2(SO3)3����д��Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ļ�ѧ����ʽ ��
�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3����ѡ��������Ϊ ������ţ�
�ò���Ӧ��Ŀ���� ��
��1����CH4������������β���е����������Ⱦ��
��֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(l) ��H����955 kJ/mol
2NO2(g)��N2O4(g) ��H����56.9 kJ/mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ ��
��2����֪��CO(g)��H2O(g)
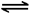 CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g)
CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g)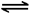 CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ��
CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ����3����ʽ��������������������Ҫ����������
����Al2(SO4)3��Һ��Ͷ���ĩ״ʯ��ʯ�����ɼ�ʽ������[Al2(SO4)3��Al2O3]��Һ��
�ڼ�ʽ����������SO2��Al2(SO4)3��Al2O3+3SO2��Al2(SO4)3��Al2(SO3)3����д��Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ļ�ѧ����ʽ ��
�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3����ѡ��������Ϊ ������ţ�
| A��Ũ���� | B��KMnO4��Һ | C��5%��H2O2��Һ | D������ |
��1��CH4(g)+N2O4(g) = N2(g) +2H2O(l) + CO2(g) ��H=" ��898.1" kJ/mol
��2��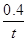 mol/( L��min) 2.25 24.6
mol/( L��min) 2.25 24.6
��3��Al2(SO4)3��Al2O3+3H2O+10NaOH=4Na[Al(OH)4]+3Na2SO4��C D������Al2(SO4)3ѭ��ʹ�á�
��2��
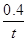 mol/( L��min) 2.25 24.6
mol/( L��min) 2.25 24.6��3��Al2(SO4)3��Al2O3+3H2O+10NaOH=4Na[Al(OH)4]+3Na2SO4��C D������Al2(SO4)3ѭ��ʹ�á�
���������(1) ����ʽH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(l) ��H����955 kJ/mol ��ȥ��ʽ2NO2(g)��N2O4(g) ��H����56.9 kJ/mol.�����ɵã�CH4(g)+N2O4(g) = N2(g) +2H2O(l) + CO2(g) ��H=" ��898.1" kJ/mol ����2���ɷ���ʽ���Կ�����ÿ����1mol��H2���ų�����41kJ�����ڷų�����32.8kJ�������H2�����ʵ���Ϊ32.8��41=0.8mol�������tmin��H2��ʾ��ƽ����Ӧ����Ϊv(H2)=��c/��t=0.8mol��2L��tmin=
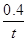 mol/( L��min). (2) ���ڷ�ӦCO(g)��H2O(g)
mol/( L��min). (2) ���ڷ�ӦCO(g)��H2O(g)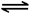 CO2(g)��H2(g)��˵�� ����Ӧ�ﵽƽ��ʱ��c(CO)=c(H2O)=(2.0-0.8)mol��2L=0.6mol/L ;c(CO2)=c(H2)=0.8mol��2L=0.4mol/L;�÷�Ӧ�Ļ�ѧƽ�ⳣ��
CO2(g)��H2(g)��˵�� ����Ӧ�ﵽƽ��ʱ��c(CO)=c(H2O)=(2.0-0.8)mol��2L=0.6mol/L ;c(CO2)=c(H2)=0.8mol��2L=0.4mol/L;�÷�Ӧ�Ļ�ѧƽ�ⳣ�� ����Ӧ CO2(g)��H2(g)
����Ӧ CO2(g)��H2(g)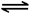 CO(g)��H2O(g)��CO(g)��H2O(g)
CO(g)��H2O(g)��CO(g)��H2O(g)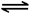 CO2(g)��H2(g)���淴Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ
CO2(g)��H2(g)���淴Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ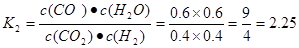 .����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2�����練Ӧ�ﵽƽ��ʱ������COΪxmol,��ˮ����Ҳ��xmol,δ��Ӧ��CO2(g)��H2(g)�����ʵ�������(1.0-x)mol.
.����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2�����練Ӧ�ﵽƽ��ʱ������COΪxmol,��ˮ����Ҳ��xmol,δ��Ӧ��CO2(g)��H2(g)�����ʵ�������(1.0-x)mol.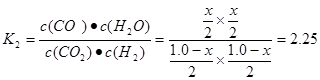 .���x=0.6mol.��˷�Ӧ�ﵽƽ������յ�����Ϊ0.6mol��41kJ/mol=24.6kJ.(3)��ΪAl2(SO4)3��Al2O3������NaOH������Ӧ�����Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ϳ��Կ�����Al2(SO4)3��Al2O3�Ļ������NaOH��Һ�����ķ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ:Al2(SO4)3��Al2O3+3H2O+10NaOH=4Na[Al(OH)4]+3Na2SO4.�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3ʱѡ������������������µ��������ӣ���������������������׳�ȥ��������Ŀ�ṩ���Լ���ѡ����ɫ������5%��H2O2��Һ����������ѡ��ΪC D���ò���Ӧ��Ŀ���ǽ�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3���Դﵽ���ʵ�ѭ�����ã���߾���Ч�档2�ķ�Ӧԭ����֪ʶ��
.���x=0.6mol.��˷�Ӧ�ﵽƽ������յ�����Ϊ0.6mol��41kJ/mol=24.6kJ.(3)��ΪAl2(SO4)3��Al2O3������NaOH������Ӧ�����Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ϳ��Կ�����Al2(SO4)3��Al2O3�Ļ������NaOH��Һ�����ķ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ:Al2(SO4)3��Al2O3+3H2O+10NaOH=4Na[Al(OH)4]+3Na2SO4.�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3ʱѡ������������������µ��������ӣ���������������������׳�ȥ��������Ŀ�ṩ���Լ���ѡ����ɫ������5%��H2O2��Һ����������ѡ��ΪC D���ò���Ӧ��Ŀ���ǽ�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3���Դﵽ���ʵ�ѭ�����ã���߾���Ч�档2�ķ�Ӧԭ����֪ʶ��
��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
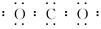
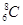

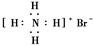
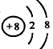 �����Ա�ʾ16O2����Ҳ���Ա�ʾ18O2��
�����Ա�ʾ16O2����Ҳ���Ա�ʾ18O2�� �����Ա�ʾ������ӣ�Ҳ���Ա�ʾ���Ȼ�̼����
�����Ա�ʾ������ӣ�Ҳ���Ա�ʾ���Ȼ�̼����