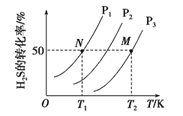
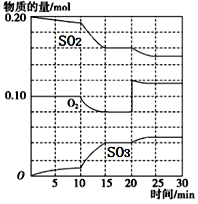
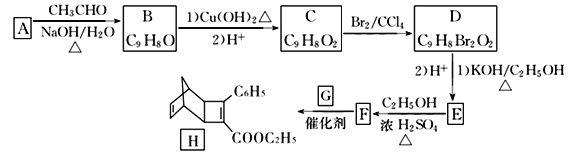
ĢāÄæÄŚČŻ
”¾ĢāÄæ”æ£Ø»Æѧє3”Ŗ”ŖĪļÖŹ½į¹¹ÓėŠŌÖŹ£©ĄūÓĆ ŗĻ³ÉµÄŠĀŠĶČżŌŖ“߻ƼĮ[La0.8Cu0.2Ni1-xMxO3(M ·Ö±šĪŖMn”¢FeŗĶCo)]æÉŅŌŹ¹Ęū³µĪ²ĘųÖŠNOŗĶCO·¢Éś·“Ó¦¶ų¼õÉŁĪ²ĘųĪŪČ¾£¬Ķ¬Ź±æÉ“ó“ó½µµĶÖŲ½šŹōµÄÓĆĮ攣»Ų“šĻĀĮŠĪŹĢā£ŗ
ŗĻ³ÉµÄŠĀŠĶČżŌŖ“߻ƼĮ[La0.8Cu0.2Ni1-xMxO3(M ·Ö±šĪŖMn”¢FeŗĶCo)]æÉŅŌŹ¹Ęū³µĪ²ĘųÖŠNOŗĶCO·¢Éś·“Ó¦¶ų¼õÉŁĪ²ĘųĪŪČ¾£¬Ķ¬Ź±æÉ“ó“ó½µµĶÖŲ½šŹōµÄÓĆĮ攣»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Mn2+µÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ£ŗ________________£¬Ę䵄µē×ÓŹżĪŖ_________________”£
(2)C”¢N”¢O”¢MnµēøŗŠŌÓɓ󵽊”µÄĖ³ŠņŹĒ___________”£
(3)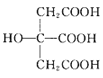 Ņ²ŹĒ³£¼ūÅäĢ壬ĘäÖŠ²ÉČ”sp2ŌӻƵÄĢ¼Ō×ÓŗĶsp3ŌӻƵÄĢ¼Ō×ÓøöŹż±ČĪŖ_____”£
Ņ²ŹĒ³£¼ūÅäĢ壬ĘäÖŠ²ÉČ”sp2ŌӻƵÄĢ¼Ō×ÓŗĶsp3ŌӻƵÄĢ¼Ō×ÓøöŹż±ČĪŖ_____”£
(4)Ą¶É«ĪļÖŹKFe(¢ó)x[Fe(¢ņ)(CN)6]æÉ»ŗ½āÖŲ½šŹōÖŠ¶¾£¬x=_______£»øĆĪļÖŹÖŠ²»“ęŌŚµÄ×÷ÓĆĮ¦ÓŠ_____”£
A£®·¶µĀ»ŖĮ¦ B£®Ąė×Ó¼ü C£®¦Ņ¼ü D£®¦Š¼ü E.Ēā¼ü
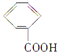
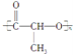
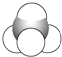
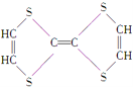
(5)ø±×åŌŖĖŲīܵÄŃõ»ÆĪļæÉŅŌŌŚŹŅĪĀĻĀĶźČ«Ńõ»Æ¼×Č©(HCHO)”£¼×Č©·Ö×ÓµÄĮ¢Ģå¹¹ŠĶĪŖ_____£»¼×Č©³£ĪĀĻĀĪŖĘųĢå¶ų¼×“¼(CH3OH)ĪŖŅŗĢåµÄŌŅņŹĒ________________________________ ”£
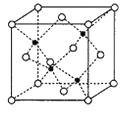
(6)ø±×åŌŖĖŲMnŗĶŌŖĖŲSeŠĪ³ÉµÄij»ÆŗĻĪļŹōÓŚĮ¢·½¾§Ļµ£¬Ę侧°ū½į¹¹ČēĶ¼ĖłŹ¾£¬ĘäÖŠ(”šĪŖSe£¬![]() ĪŖMn)£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ________£¬SeµÄÅäĪ»ŹżĪŖ______£¬MnŗĶSeµÄĦ¶ūÖŹĮæ·Ö±šĪŖM1g/mol”¢M2g/mol£¬øĆ¾§ĢåµÄĆܶČĪŖ¦Ńg/cm3,ŌņMn”ŖSe¼üµÄ¼ü³¤ĪŖ_____________nm(¼ĘĖć±ķ“ļŹ½)”£
ĪŖMn)£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ________£¬SeµÄÅäĪ»ŹżĪŖ______£¬MnŗĶSeµÄĦ¶ūÖŹĮæ·Ö±šĪŖM1g/mol”¢M2g/mol£¬øĆ¾§ĢåµÄĆܶČĪŖ¦Ńg/cm3,ŌņMn”ŖSe¼üµÄ¼ü³¤ĪŖ_____________nm(¼ĘĖć±ķ“ļŹ½)”£
”¾“š°ø”æ1s22s22p63s23p63d5»ņÕß[Ar]3d5 5 O£¾N£¾C£¾Mn 1:1 1 AE Ę½ĆęČż½ĒŠĪ ¼×“¼æÉŅŌŠĪ³É·Ö×Ó¼äĒā¼ü MnSe 4 ![]()
”¾½āĪö”æ
(1)ĆĢŹĒ25ŗÅŌŖĖŲ£¬Mn2+µÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d5£¬ĘäÖŠ3d5ÖŠµÄ5øöµē×Ó·Ö²¼ŌŚ5øöŌ×Ó¹ģµĄÖŠ£¬Ņņ“Ėµ„µē×ÓŹżĪŖ5£¬¹Ź“š°øĪŖ£ŗ1s22s22p63s23p63d5£»5£»
(2)ŌŖĖŲµÄ½šŹōŠŌŌ½Ē棬µēøŗŠŌŹżÖµŌ½Š”£¬·Ē½šŹōŠŌŌ½Ē棬µēøŗŠŌŹżÖµŌ½“ó£¬C”¢N”¢O”¢MnµēøŗŠŌÓɓ󵽊”µÄĖ³ŠņŹĒO£¾N£¾C£¾Mn£¬¹Ź“š°øĪŖ£ŗO£¾N£¾C£¾Mn£»
(3) Ņ²ŹĒ³£¼ūÅäĢ壬ĘäÖŠC=OÖŠµÄĢ¼Ō×Ó²ÉČ”sp2Ōӻƣ¬¹²3øö£¬ĘäÓąĢ¼Ō×Ó²ÉÓĆsp3Ōӻƣ¬¹²3øö£¬øöŹż±ČĪŖ1:1£¬¹Ź“š°øĪŖ£ŗ1:1£»
Ņ²ŹĒ³£¼ūÅäĢ壬ĘäÖŠC=OÖŠµÄĢ¼Ō×Ó²ÉČ”sp2Ōӻƣ¬¹²3øö£¬ĘäÓąĢ¼Ō×Ó²ÉÓĆsp3Ōӻƣ¬¹²3øö£¬øöŹż±ČĪŖ1:1£¬¹Ź“š°øĪŖ£ŗ1:1£»
(4)øł¾ŻÕżøŗ»ÆŗĻ¼Ū“śŹżŗĶĪŖ0£¬KFe(III) x[Fe(II)(CN)6]ÖŠ“ęŌŚ(+1)+(+3)”Įx+(+2)+(-1)”Į6=0£¬½āµĆx=1£¬øĆĪļÖŹŹōÓŚÅäŗĻĪļ£¬ĘäÖŠ“ęŌŚµÄ×÷ÓĆĮ¦ÓŠĄė×Ó¼ü”¢¦Ņ¼ü”¢CN-ÖŠ“ęŌŚ¦Š¼ü£¬ŅŌ¼°ÅäĪ»¼ü£¬²»“ęŌŚ·¶µĀ»ŖĮ¦ŗĶĒā¼ü£¬¹ŹŃ”AE£¬¹Ź“š°øĪŖ£ŗ1£»AE£»
(5)¼×Č©·Ö×ÓÖŠµÄĢ¼Ō×Ó3øöŌ×ÓĻąĮ¬£¬Ć»ÓŠ¹Ā¶Ōµē×Ó£¬²ÉÓĆsp2Ōӻƣ¬Į¢Ģå¹¹ŠĶĪŖĘ½ĆęČż½ĒŠĪ£»¼×“¼æÉŅŌŠĪ³É·Ö×Ó¼äĒā¼ü£¬¶ų¼×Č©²»ÄÜ£¬Ņņ“Ė¼×Č©³£ĪĀĻĀĪŖĘųĢå¶ų¼×“¼(CH3OH)ĪŖŅŗĢ壬¹Ź“š°øĪŖ£ŗĘ½ĆęČż½ĒŠĪ£»¼×“¼æÉŅŌŠĪ³É·Ö×Ó¼äĒā¼ü£»
(6)øł¾Ż¾§°ū½į¹¹Ķ¼£¬SeŌ×ÓøöŹżĪŖ8”Į![]() +6”Į
+6”Į![]() =4£¬MnŌ×ÓøöŹż=4£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖMnSe£»ĆæøöSeÖÜĪ§ÓŠ4øöMnŌ×Ó¾ąĄė×ī½üĒŅĻąµČ£¬SeµÄÅäĪ»ŹżĪŖ4£»1mol¾§°ūµÄÖŹĮæĪŖ(4M1+4M2)g£¬Éč¾§°ūµÄ±ß³¤ĪŖxcm£¬1mol¾§°ūµÄĢå»żĪŖNA”Įx3cm3£¬Ōņx=
=4£¬MnŌ×ÓøöŹż=4£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖMnSe£»ĆæøöSeÖÜĪ§ÓŠ4øöMnŌ×Ó¾ąĄė×ī½üĒŅĻąµČ£¬SeµÄÅäĪ»ŹżĪŖ4£»1mol¾§°ūµÄÖŹĮæĪŖ(4M1+4M2)g£¬Éč¾§°ūµÄ±ß³¤ĪŖxcm£¬1mol¾§°ūµÄĢå»żĪŖNA”Įx3cm3£¬Ōņx=![]() cm£¬Mn”ŖSe¼üµÄ¼ü³¤ĪŖ¾§°ūĢå¶Ō½ĒĻß³¤¶ČµÄ
cm£¬Mn”ŖSe¼üµÄ¼ü³¤ĪŖ¾§°ūĢå¶Ō½ĒĻß³¤¶ČµÄ![]() £¬ŌņMn”ŖSe¼üµÄ¼ü³¤=
£¬ŌņMn”ŖSe¼üµÄ¼ü³¤=![]() ”Į
”Į![]() cm=
cm=![]() ”Į
”Į![]() ”Į107nm£¬¹Ź“š°øĪŖ£ŗMnSe£»4£»
”Į107nm£¬¹Ź“š°øĪŖ£ŗMnSe£»4£»![]() ”Į
”Į![]() ”Į107”£
”Į107”£

 Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø