��Ŀ����
����Ŀ���о�������NOx��SO2����������Ҫ�ɷ֡�
��NOx��Ҫ��Դ������β����
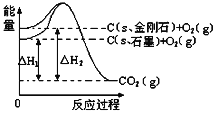
��֪��N2��g����O2��g��![]() 2NO��g�� ��H����180.50 kJ��mol��1
2NO��g�� ��H����180.50 kJ��mol��1
2CO��g����O2��g��![]() 2 CO2��g�� ��H����566.00kJ��mol��1
2 CO2��g�� ��H����566.00kJ��mol��1
��1��Ϊ�˼��������Ⱦ���������������β�������ܿڲ��ô�����NO��COת��������Ⱦ����������ѭ����д���÷�Ӧ���Ȼ�ѧ����ʽ_________________��
��2��T��ʱ���������ʵ�����NO��CO�����ݻ�Ϊ2L���ܱ������У������¶Ⱥ�������䣬��Ӧ���̣�0��15min����NO�����ʵ�����ʱ��仯�Ĺ�ϵ����ͼ��ʾ��
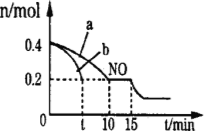
��T��ʱ�û�ѧ��Ӧ��ƽ�ⳣ��K��_______________��ƽ��ʱ�������¶Ȳ��䣬���������г���CO��N2��0.8mol��ƽ�⽫_______�ƶ���������������ҡ�����
��ͼ��a��b�ֱ��ʾ��һ���¶��£�ʹ��������ͬ���������ͬ�Ĵ���ʱ���ﵽƽ�������n(NO)�ı仯���ߣ����б�ʾ����������ϴ��������__________���a����b������
��15minʱ�����ı���練Ӧ����������n(NO)������ͼ��ʾ�ı仯����ı������������_______��
����SO2��Ҫ��Դ��ú��ȼ�ա�ȼú��������������Ǽ��ٴ����к�������Ⱦ�Ĺؼ���
��3���ô�����Һ����SO2�ɽ���ת��ΪHSO3�����÷�Ӧ�����ӷ���ʽ��___________________��
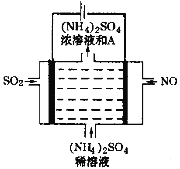
��4����ͼ���װ�ÿɽ������е�NO��SO2�ֱ�ת��ΪNH4+��SO42��������A�Ļ�ѧʽΪ_____________�������ĵ缫��Ӧʽ��________________________��
���𰸡���1��2NO��g��+2CO��g��![]() 2CO2��g��+N2��g����H=��746.50kJ��mol��1
2CO2��g��+N2��g����H=��746.50kJ��mol��1
��2����5mol/L-1�����ң�2�֣�����b��������CO�����ʵ���Ũ�ȣ�������ѹǿ�������¶ȵ���
��3��H2O+2SO2+CO32-=2HSO3-+CO2����2����
��4��H2SO4��1�֣���NO+6H++5e��=NH4++H2O��2�֣�
��������
�����������1����֪��N2(g)+O2(g)![]() 2NO(g)��H=+180.50kJmol-1��
2NO(g)��H=+180.50kJmol-1��
2CO(g)+O2(g)![]() CO2(g)��H=-566.00kJmol-1��
CO2(g)��H=-566.00kJmol-1��
���ݸ�˹���ɣ���-�ٵõ���2NO(g)+2CO(g)![]() 2CO2(g)+N2(g)��H=-746.50 kJmol-1��
2CO2(g)+N2(g)��H=-746.50 kJmol-1��
��2������ʼʱ��NOΪ0.4mol��ƽ��ʱNOΪ0.2mol��
2NO(g)+2CO(g)![]() 2CO2(g)+N2
2CO2(g)+N2
��ʼ����0.4mol 0.4mol 0 0
ת������0.2mol 0.2mol 0.2mol 0.1mol
ƽ������0.2mol 0.2mol 0.2mol 0.1mol
��ƽ��ʱ��Ũ�ȣ�c(NO)=0.1mol/L��c(CO)=0.1mol/L��c(CO2)=0.1mol/L��c(N2)=0.05mol/L
k��![]() ��5(mol/L)-1��
��5(mol/L)-1��
ƽ��ʱ�������¶Ȳ��䣬���������г���CO��N2��0.8mol��
��c(CO)=0.5mol/L��c(N2)=0.45mol/L��
Qc��![]() ��1.8��k����ƽ�⽫�����ƶ���
��1.8��k����ƽ�⽫�����ƶ���
�ڴ���������ϴ�Ӧ���ʿ죬�ﵽƽ������ʱ��̣���ͼ��֪��b���ߴ����������·�Ӧ���ʿ죬����b�Ĵ����ı�����ʴ�Ϊb��
����ͼ���֪��NO��Ũ�ȼ�С��ƽ�����������ƶ������Ըı������Ϊ����CO�����ʵ���Ũ�Ȼ�����ѹǿ��
��3��̼������Һ��SO2��Ӧ�������������ƺͶ�����̼���䷴Ӧ�����ӷ���ʽΪ��H2O+2SO2+CO32-��2HSO3-+CO2����
��4�����װ�D�ɽ������е�NO��SO2�ֱ�ת��ΪNH4+��SO42-�����ⷽ��ʽΪ5SO2+2NO+8H2O![]() (NH4)2SO4+4H2SO4���ɵ�ⷽ��ʽ��֪������AΪ���ᣬ��Ļ�ѧʽH2SO4�����ʱ�������϶�������ʧ����������������ӣ�������NO�õ�����ת��Ϊ笠����������ĵ缫��Ӧʽ��NO + 6H+ + 5e��= NH4+ +H2O��
(NH4)2SO4+4H2SO4���ɵ�ⷽ��ʽ��֪������AΪ���ᣬ��Ļ�ѧʽH2SO4�����ʱ�������϶�������ʧ����������������ӣ�������NO�õ�����ת��Ϊ笠����������ĵ缫��Ӧʽ��NO + 6H+ + 5e��= NH4+ +H2O��

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�