��Ŀ����
����Ԫ��X��Y��Z��W��ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���ֻ�������ܼ����Ҹ��ܼ���������� |
| Y | Y�Ļ�̬ԭ�����������������ڲ����������2.5�� |
| Z | Z�Ļ�̬�۵��ӽṹΪnsn-1 |
| W | W���ʳ��ڻ�ɽ�ڸ��������֣���������������������Ҫԭ��֮һ |
��1��Yλ��Ԫ�����ڱ��� ���� �壬���̬ԭ��δ�ɶԵ����� ����
��2��X�ĵ縺�Ա�W�� �����С������Y�������̬�⻯���X�������̬�⻯����Һ��������Ҫԭ���� ��
��3��Z��ͬ�����������ڵ�����Ԫ�ص�ԭ����Ƚϣ����ߵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ����Y��Z�γɵĻ�����Ϊ ���壬��������ˮǿ��ˮ��Ļ�ѧ����ʽΪ ��
��4����һ���¶��£���һ���ݻ�������ܱ������г���1molY2��3mol������������Ӧ��Y2(g)+3H2(g) 2YH3(g) ��H=-akJ/mol���ڸ������´ﵽƽ��ʱ�ų�������ΪbkJ����ƽ�ⳣ������ʽK= ������ʼ��������г���2molYH3������ͬ�¶��´ﵽƽ��ʱ����Ӧ���������յ�����ΪckJ����a��b��c����֮��Ĺ�ϵΪ ����һ��ʽ�ӱ�ʾ����
��1��2��VA��3 ����1�֣���3�֣�
��2��С��������֮������γ���� ����1�֣�2�֣�
��3��Mg��Al��Na�����ӣ�Mg3N2+6H2O=3Mg(OH)2+2NH3�� ����2�֣�6�֣�
��4��c2(NH3)/[c(N2)c3(H2)] a=b+c ����2�֣�4�֣�
���������������������֪X��Y��Z��W�ֱ���C��N��Mg��S��
Nλ��Ԫ�����ڱ��ĵڶ����ڵ�VA�壬���̬ԭ�Ӻ�������Ų�ʽΪ1S22S2P3������3��δ�ɶԵ��ӣ�
C�ĵ縺�Ա�S��ҪС��NH3��CH4��Һ������Ϊ�����Ӽ���������
����Ԫ��������֪ͬһ����Ԫ�ش����ҵ�һ������������þ���������3S2����ȫ�����ṹ�����ȶ�����һ�����ܽϺ������Ҫ��Mg3N2�����Ӿ��壬��ˮ��Ӧ�ķ���ʽ��Mg3N2+6H2O=3Mg(OH)2+2NH3����
��ҵ�ϳɰ���ƽ�ⳣ������ʽ��K= c2(NH3)/[c(N2)c3(H2)]�����ݿ��淴Ӧ���ص�֪a��b��c����֮��Ĺ�ϵ��a=b+c��
���㣺������Ԫ���ƶ�Ϊ����������ԭ�ӽṹ���������͡������Ԫ�������ɡ�Ԫ�ؼ��������ѧƽ�ⳣ�����Ȼ�ѧ����ʽ���ص��֪ʶ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���A��B��C��D��E��F���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���
| Ԫ�ر�� | Ԫ��������Ϣ |
| A | A�ĵ������ܶ���С������ |
| B | B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ������������ |
| C | C��ԭ�����������������ڲ������������ |
| D | D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С |
| E | B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ� |
| F | FԪ���������������۵Ĵ�����Ϊ4 |
��2��D��E��F�ļ����Ӱ뾶�ɴ�С��˳����(ֱ���û�ѧʽ��ʾ) ��
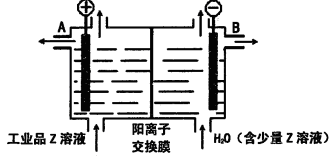
��3����Fe��D������ɵĻ�����У���������F������������Ӧˮ�����ϡ��Һ������ȫ���ܽ⡣�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȡ���ԭ�������D���ʵ���������
Ϊ ��
��4��һ������ʯ������ͨ��һ������E���ʣ�����ǡ����ȫ��Ӧ���������������ֺ�EԪ�ص����ӣ������������ӵ����ʵ���(n)�뷴Ӧʱ��(t)��������ͼ��ʾ����ʱ��Ӧ�Ļ�ѧ����ʽΪ ��
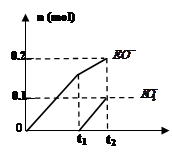
��5��A��B�γɵĻ�����BA���л��ϳ�����;�ܹ㷺�������Զ�ȡ�ܶ�����е����Ӷ�������Ӧ���ƵĻ����д�������Ҵ���Ӧ�Ļ�ѧ����ʽ ��
A��B��C��D��E�����ڱ���ǰ�����ڵ�Ԫ�أ����й����ʻ�ṹ��Ϣ���±���
| Ԫ�� | �й����ʻ�ṹ��Ϣ |
| A | �����۵�AԪ�ص��⻯����ͨ��״������һ��Һ�壬����A����������Ϊ88.9�� |
| B | Bԭ�ӵõ�һ�����Ӻ�3p���ȫ���� |
| C | Cԭ�ӵ�p����������������̬�⻯������������������ˮ���ﷴӦ����һ�ֳ�������X |
| D | DԪ�ص�����ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣬�����������Ϊ���Ӿ��� |
| E | EԪ�صĺ˵��������Aԭ�Ӻ�BԪ���⻯��ĺ˵����֮�� |
(2)EԪ��ԭ�ӵĺ�������Ų�ʽΪ
(3)��X��ˮ��Һ�� (����ԡ��������ԡ������ԡ�)��BԪ����ۺ�����һ����DԪ����ۺ���������� (�ǿ��������)��
(4)C���ʷ����ЦҼ��ͦм��ĸ�����Ϊ ��C���⻯����ͬ��Ԫ�ص��⻯���зе���ַ�������ԭ����
(5)�ø�����������Һ̬H2Aʱ��һ��H2A�������ͷų�һ�����ӣ�ͬʱ����һ�־��н�ǿ�����Ե������ӣ���д���������ӵĵ���ʽ�� ��д����������������⻯���ˮ��Һ��Ӧ�����ӷ���ʽ��
��14�֣�Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���������������Ϣ���ɣ������ǰ��ֶ�����Ԫ�ص������Ϣ����֪���ԭ�Ӱ뾶Ϊ0.089nm��
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -2 |
��1��BԪ����Ԫ�����ڱ��е�λ��___________________����������Ԫ�ص�����������Ӧ��ˮ������������ǿ����__________��A���ӵĽṹʾ��ͼ_______________��
��2���õ���ʽ��ʾA��D�γɻ�����Ĺ��̣�____________________________________��H��E�γ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽΪ___________�����еĻ�ѧ������Ϊ____________��C2D2�ĵ���ʽΪ______________________��
��3������˵����˵��D�ķǽ����Ա�Cǿ��ѡ��____________
��H2CO4��HDO�ȶ���HDO4��H2CO4����ǿ��C2-��D-�ױ�������HD��H2C�ȶ���ͭ��HD����Ӧ��������ŨH2CO4��Ӧ������D2��������FeD3������C��������FeC��Cԭ����Dԭ�ӵ��Ӳ�����ͬ��Dԭ�Ӱ뾶С��Cԭ�ӡ�
A��ȫ�� B���ڢۢܢޢ� C���٢ڢܢݢ� D����������
��4��A��B��C��D��E�γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________________�����þ������ӷ��ű�ʾ��
��5��C������H������������Ӧˮ�����ڼ����������ܷ�����Ӧ������3mol��C���뷴Ӧ��ת��4NA�ĵ��ӣ���д�����ӷ�Ӧ����_______________________________________���������뻹ԭ��������֮��_____________________��

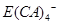 ����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.
����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.