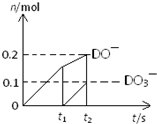
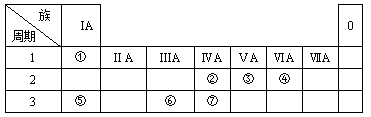
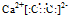��Ŀ����
���л�ѧ�������ر�����ȷ���У�������
������A������NaOH��������ˮҪ���ȣ�
B��������ˮ����ͭ�ܽ�Ϊ��Һʱ���ȷ��ȱ����ˮ����ͭ����ˮ����ͭ�����������������ձ���Ϊ���ȣ��ų������������������仯֮�
C��ȼ������ָ1 mol������ȫȼ�������ȶ�������ʱ���ų�������������ȼ�շ�Ӧ�Ƿ��ȷ�Ӧ�����������ȷ�Ӧ��
D������N2��g��+3H2��g��?2NH3Ϊ���淴Ӧ�����ܽ��е��ף�
B��������ˮ����ͭ�ܽ�Ϊ��Һʱ���ȷ��ȱ����ˮ����ͭ����ˮ����ͭ�����������������ձ���Ϊ���ȣ��ų������������������仯֮�
C��ȼ������ָ1 mol������ȫȼ�������ȶ�������ʱ���ų�������������ȼ�շ�Ӧ�Ƿ��ȷ�Ӧ�����������ȷ�Ӧ��
D������N2��g��+3H2��g��?2NH3Ϊ���淴Ӧ�����ܽ��е��ף�
����⣺A��NaOH��������ˮҪ���ȣ���1 mol NaOH������������ϡ���ᷴӦ���ų�����������a kJ����A����
B��CuSO4?5H2O��s���TCuSO4��s��+5H2O��l����H=+b kJ/mol����1 mol CuSO4��s����ˮ����CuSO4?5H2O���ų�bkJ���������ɵ�CuSO4?5H2O����ˮʱ��Һ���ȣ�����1 mol CuSO4��s������ˮʱ���ų������������������仯֮�С��b kJ��������B����
C��ȼ������ָ1 mol������ȫȼ�������ȶ�������ʱ���ų���������������ȼ����Ϊc kJ/mol���ʵ��ˮ���Ȼ�ѧ����ʽΪ2H2O��l���T2H2��g��+O2��g����H=+2c kJ/mol����C����
D������N2��g��+3H2��g��?2NH3Ϊ���淴Ӧ�����ܽ��е��ף�����ij������ͨ��1 mol N2��3 mol H2��ַ�Ӧ�ų�������С��d kJ����D��ȷ��
��ѡD��
B��CuSO4?5H2O��s���TCuSO4��s��+5H2O��l����H=+b kJ/mol����1 mol CuSO4��s����ˮ����CuSO4?5H2O���ų�bkJ���������ɵ�CuSO4?5H2O����ˮʱ��Һ���ȣ�����1 mol CuSO4��s������ˮʱ���ų������������������仯֮�С��b kJ��������B����
C��ȼ������ָ1 mol������ȫȼ�������ȶ�������ʱ���ų���������������ȼ����Ϊc kJ/mol���ʵ��ˮ���Ȼ�ѧ����ʽΪ2H2O��l���T2H2��g��+O2��g����H=+2c kJ/mol����C����
D������N2��g��+3H2��g��?2NH3Ϊ���淴Ӧ�����ܽ��е��ף�����ij������ͨ��1 mol N2��3 mol H2��ַ�Ӧ�ų�������С��d kJ����D��ȷ��
��ѡD��
���������⿼���������ܽ�����е������仯����˹���ɵ������Լ����淴Ӧ����������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ