��Ŀ����
| Ԫ�� | �����Ϣ |
| X | XԪ���ڿ����к������ |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
| Z | Z��Yͬ���壬ԭ�Ӱ뾶��Y��Z |
| W | WԪ�ص�һ����������д��� |
| H | HԪ���γɵ�����Һһ�����ɫ |
��1������ѡ��������Ϊ�ж�Y��Z �ķǽ�����ǿ���������У�����ĸ��ţ�
a��Y��Z�ĵ��ʵ��۵�ߵ�
b��Y��Z���⻯����ȶ���ǿ��
c��Y��Z���⻯��ķе�ߵ�
d��Y��Z������������Ӧ��ˮ���������ǿ��
��2���õ���ʽ��ʾX������⻯����γɹ���
��3��YԪ����Ԫ�����ڱ��е�λ��Ϊ
��4��ȡ100mL1mol/L��3���� A����Һ������W���ʺ�H���ʵĻ�����ĩ��6g��ֽ��������ȫ�ܽ⣬����Ӧ����Һ�м���KSCN��Һ�������������������ĩ��W���ʵ���������Ϊ
��5��ȡ100mL���ʵ���Ũ�Ⱦ�Ϊ1mol/L��X����ۺ������Y����ۺ�����Ļ��ϡ��Һ����������H���ʵķ�ĩ��ֽ��裬������ܽ�÷�ĩ
��1��a��Y��Z�ĵ��ʵ��۵�ߵͣ������������ʣ����ܱȽϷǽ�����ǿ������a����
b��Ԫ�صķǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�����b��ȷ��
c��Y��Z���⻯��ķе�ߵͣ������������ʣ����ܱȽϷǽ�����ǿ������c����
d��OԪ��û�ж�Ӧ���ᣬ����������������Ӧ��ˮ���������ǿ���Ƚ���ǽ�����ǿ������d����
�ʴ�Ϊ��b��
��2���õ���ʽ��ʾNH3���γɹ���Ϊ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3��YΪSԪ�أ�ԭ����3�����Ӳ㣬����������Ϊ6����Ԫ�����ڱ��е�λ��Ϊ�������ڵڢ�A�壮������A��S��O��Fe����Ԫ����ɣ���ˮ��Һ�ܹ��ܽ�Fe�ĵ��ʣ�������AΪFe2��SO4��3������Һ��Fe3+ˮ�⣺Fe3++3H2O?Fe��OH��3�����壩+3 H+�����������������壬�ܹ���ˮ��
�ʴ�Ϊ���������ڵڢ�A�壻Fe3++3H2O?Fe��OH��3�����壩+3H+��
��4��ȡ100mL 1mol/LFe2��SO4��3����Һ������Fe���ʺ�Cu���ʵĻ�����ĩ��6g��ֽ��������ȫ�ܽ⣬����Ӧ����Һ�м���KSCN��Һ������������˵��Fe2��SO4��3�����ǡ�÷�Ӧ��ȫ��Ӧ����Һ������ΪFeSO4��CuSO4��
Fe2��SO4��3�����ʵ���=0.1L��1mol/L=0.1mol����Fe��Cu�����ʵ����ֱ�Ϊxmol��ymol������FeԪ���غ㣬����Һ�������������ʵ���=��x+0.2��mol���ɵ���غ��֪��2��x+0.2��+2y=0.1��3��2�����ݽ��������ɵ�56x+64y=6���������̽�ã�x=0.05��y=0.05��
��Fe����������=
| 0.05mol��56g/mol |
| 6g |
�ʴ�Ϊ��46.7%��
��5��ȡ100mL���ʵ���Ũ�Ⱦ�Ϊ1mol/L�����ᡢ����Ļ��ϡ��Һ�������Һn��H+��=0.1L��1mol/L+2��0.1L��1mol/L=0.3mol��n��NO3-��=0.1L��1mol/L=0.1mol����
3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O
0.1mol 0.4mol
0.1molNO3-��ȫ��Ӧ��Ҫ0.4molH+��H+���㣬�ʸ���H+�����ܽ��Cu���ɷ���ʽ��֪����ܽ�Cu�����ʵ���=0.3mol��
| 3 |
| 8 |
| 3 |
| 8 |
�ʴ�Ϊ��7.2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���14�֣�X��Y��Z��W��Q��ԭ��������������Ķ���������Ԫ�أ������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | Xԭ�Ӻ��������������Ǵ�����2�� |
| Y | Y����̬�⻯���ˮ��Һ�������� |
| Z | Z�ǵؿ��к������Ľ���Ԫ�� |
| W | ���³�ѹ�£�W�ĵ����ǵ���ɫ���� |
| Q | ���� |
 (1)Ԫ��Q��Ԫ�����ڱ��е�λ��______________________________��
(1)Ԫ��Q��Ԫ�����ڱ��е�λ��______________________________��(2)Y�������̬�⻯���ˮ��Һ����H2O2������Ӧ������ﲻ��Ⱦ��������ѧ����ʽΪ______________________________________������Ԫ�ط��ű�ʾ����ͬ��
(3)X����Ԫ����ɵĻ����������6��ԭ�ӣ���ṹʽΪ ��
(4)��֪��X(s)+O2(g) =XO2(g) ��H = -393.5��J��mol-1
2X(s)+O2(g) ="2XO(g) " ��H = -221.0��J��mol-1
��XO��ȼ���ȵ��Ȼ�ѧ����ʽ__________________________________________________.
(5)Ԫ��Y����Ԫ���γɵ�����������ң����Һ�Z�������ӵĻ����Һ�м������Na2O2������Na2O2�����ʵ����������������ͼ��ʾ��ϵ��
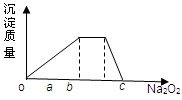
д���йط�Ӧ���ӷ���ʽ��(ÿ��ֻ��һ�����ӷ���ʽ��ʾ)
o��a��
a��b�� ��
X��Y��Z��W��T��ԭ���������������ǰ�ĸ����ڵ�Ԫ�أ������������ǽ���Ԫ�ء��������Ϣ���±���
Ԫ�� | �����Ϣ |
X | Xһ�ֺ����ڿ���ʱ�������ᶨһЩ�������� |
Y | Y��̬ԭ�ӵ�s���������P������������ |
Z | Z���������ڵĵ��������а뾶��С |
W | W�ĵ��ʱ���Ϊ����Ϣ�����Ĵ������������뵼����� |
T | T�ж��ֻ�������ɫ���������ڿ����л�Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ |
��1��X��Y��Z����Ԫ�صĵ縺���ɴ�С��˳����________����Ԫ�ط��ű�ʾ����ͬ������H-X��H-Y���ֹ��ۼ��У����ļ��Խϴ����??? ????????????? ��
��2��T2+�ĵ����Ų�ʽΪ_________��T�ĵ����ڸ�������Y���⻯�ﷴӦ���仯ѧ����ʽΪ??????????????? ����ҵ����W����������X�ĵ��ʸ����·�Ӧ�Ƶ�W���ʵĴֲ�Ʒ���仯ѧ��Ӧ����ʽΪ ?? ?????????????????????????
��3�������⻯��X2H2��H2Y2�е����ܴ���е�ϸߵ���_______________�����߷е����ܴ��ԭ����____________________________________��
��4����25����101 kPa�£���֪W�ȶ�����̬�⻯����Y����̬��������ȫȼ�գ��ָ���ԭ��״̬��ƽ��ÿ����4gW�ȶ�����̬�⻯�����190.0KJ����÷�Ӧ���Ȼ�ѧ����ʽΪ _____________��