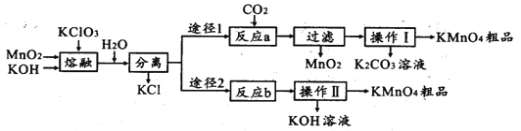
��Ŀ����
����Ŀ������β���к���CO��NOx���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת��Ϊ�����塣
��1����֪ 4CO(g)��2NO2(g)![]() 4CO2(g)��N2(g) ��H����1200 kJ��mol1
4CO2(g)��N2(g) ��H����1200 kJ��mol1
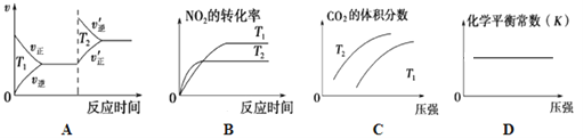
�ٸ÷�Ӧ��________________���������¡����»��κ��¶����������Է����С�
�ڶ��ڸ÷�Ӧ���ı�ijһ��Ӧ�������¶�T1>T2��������ͼ����ȷ����_______(�����)��
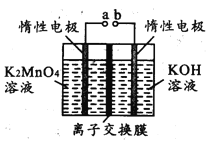
��ijʵ��С��ģ�������������̣�һ���¶��£���2L�ĺ����ܱ������У���ʼʱ���ռס������ַ�ʽ����Ͷ�ϣ�����һ��ʱ���ﵽƽ��״̬����ü���CO��ת����Ϊ50%����÷�Ӧ��ƽ�ⳣ��Ϊ__________�����ַ�ʽ��ƽ��ʱ��N2�������������______�ң� ����>��=��<��ȷ��������ͬ����NO2��Ũ�ȣ���______�ҡ�

��2����������β���е�̼��(C)��NOx��ͨ��ij���ܴ�������������ͬ�¶��£���ģ��β�����ɷ����±���ʾ������ͬ������ͨ���ô���������в���(CO2��N2��N2O)��NO��������ݽ����ͼ��ʾ��
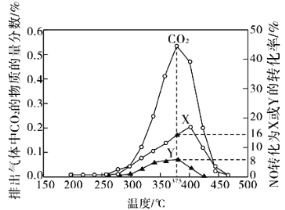
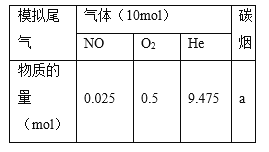
��375��ʱ������ų��������к�0.45 mol O2��0.0525 mol CO2����Y�Ļ�ѧʽΪ________��
��ʵ������в���NOģ��NOx����������NO2��ԭ����__________________��
���𰸡����� CD 10 > > N2O 2NO2![]() N2O4��NO2�����д���N2O4�������ڶ����ⶨ��
N2O4��NO2�����д���N2O4�������ڶ����ⶨ��
��������
�Ÿ÷�Ӧ��S < 0����H < 0��������G = ��H��T��S< 0�Ľ��ۣ����ݻ�ѧƽ���ƶ���˼����������������˼ά�����м��㣬�ý�ģ˼��˼����ѹƽ���ƶ���Ũ�ȱ仯��
����ģ��β����һ�����������ʵ���0.025mol��ģ��β����O2�����ʵ���Ϊ0.5mol������ų��������к�0.45 mol O2��˵��ʵ�ʲ��뷴Ӧ�����������ʵ���Ϊ0.05mol��ͬʱ���0.0525 mol CO2����ͼ�в��뷴Ӧ����X��Y��һ�����������ʵ���Ϊ��0.025mol����8%+16%��=0.006mol���������غ㣬����һ�������������ʵ���Ϊ��0.05��2+0.006-0.0525��2=0.001mol�����ݵ��غ��֪���������ʵ���Ϊ���м���ó��˽��ۡ�
��NO2��Ҫ����2NO2![]() N2O4�ķ�Ӧ�������ڶ����ⶨ��
N2O4�ķ�Ӧ�������ڶ����ⶨ��
�����÷�Ӧ��S < 0����H < 0����G = ��H��T��S< 0����Ӧ�ڵ��������Է����У��ʴ�Ϊ�����¡�
��Aѡ����£������淴Ӧ���ʶ����ߣ���A����
Bѡ������ȹ���ƽ�⣬���ִ���T2 > T1����B����
Cѡ���ѹ��ƽ�������ƶ���������̼�����ӣ������������һ����y���ƽ���ߣ����µ��ϣ����£�ƽ������ȷ����ƶ��������ƶ�����C��ȷ��
Dѡ���ѹ��ƽ�ⳣ�����䣬ƽ�ⳣ��ֻ���¶��йأ���D��ȷ��
�����������𰸰�ΪCD��
��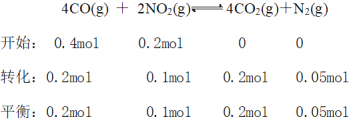
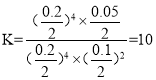 ����÷�Ӧ��ƽ�ⳣ��Ϊ10�����൱��2���������ң�����С�����������ѹ��ƽ�������ƶ���N2���ӣ���������������N2������������� > �ң�����NO2��Ũ��������2�������ϼ��٣�ƽ���ƶ������ģ����NO2��Ũ�ȣ��� > �ң��ʴ�Ϊ��10�� >��>��
����÷�Ӧ��ƽ�ⳣ��Ϊ10�����൱��2���������ң�����С�����������ѹ��ƽ�������ƶ���N2���ӣ���������������N2������������� > �ң�����NO2��Ũ��������2�������ϼ��٣�ƽ���ƶ������ģ����NO2��Ũ�ȣ��� > �ң��ʴ�Ϊ��10�� >��>��
����ģ��β����һ�����������ʵ���0.025mol��ģ��β����O2�����ʵ���Ϊ0.5mol������ų��������к�0.45 mol O2��˵��ʵ�ʲ��뷴Ӧ�����������ʵ���Ϊ0.05mol��ͬʱ���0.0525 mol CO2����ͼ�в��뷴Ӧ����X��Y��һ�����������ʵ���Ϊ��0.025mol����8%+16%��=0.006mol���������غ㣬����һ�������������ʵ���Ϊ��0.05��2+0.006-0.0525��2=0.001mol�����ݵ��غ��֪���������ʵ���Ϊ��![]() ������16%��Ӧ���ǵ�������8%��Ӧ��һ�����������ʴ�Ϊ��N2O��
������16%��Ӧ���ǵ�������8%��Ӧ��һ�����������ʴ�Ϊ��N2O��
��ʵ������в���NOģ��NOx����������NO2��ԭ����2NO2![]() N2O4��NO2�����д���N2O4�������ڶ����ⶨ���ʴ�Ϊ��2NO2
N2O4��NO2�����д���N2O4�������ڶ����ⶨ���ʴ�Ϊ��2NO2![]() N2O4��NO2�����д���N2O4�������ڶ����ⶨ��
N2O4��NO2�����д���N2O4�������ڶ����ⶨ��

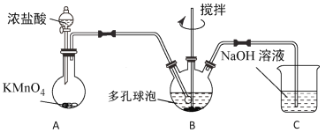
����Ŀ��ij��ѧ��ȤС�����к͵ζ����ⶨһƿ�ռ���Һ��Ũ�ȣ����ռ���Һ�в������ᷴӦ�����ʣ����ⶨ�������£�
a.����ƿ������ʽ�ζ����£���ƿ�µ�һ�Ű�ֽ��
b.�ֱ�ȡ25.00mL�ᡢ��ζ��ܸ�һ֧��ϴ�Ӳ��ô�װҺ��ϴ��
c.����ʽ�ζ���������ʼ�ζ�ֱ���յ㣬��¼���յ�ʱ�ζ����ϵĶ�����
d.ȡһ��������ˮϴ������ƿ���Ӽ�ʽ�ζ����зų�20.00mL�����Һ����ƿ�У�����2��3�η�̪��Һ��
e.��ʽ�ζ����м�������ռ���Һ����ʽ�ζ����м���0.1000mol��L-1��ϡ�������Һ��������֧�ζ��ܼ��첿�ֵ����ݾ��Ͼ�������Һ��̶ȣ�
f.���ظ���������2�Ρ�
���βⶨ��õ��������±���
ʵ����� | ����Һ�����mL�� | �����������mL�� | |
�ζ�ǰ���� | �ζ������ | ||
1 | 20.00 | 0.45 | 24.40 |
2 | 20.00 | 2.20 | 26.25 |
3 | 20.00 | 0.10 | 17.10 |
��1������ʵ���������ȷ˳����___������ĸ��ţ���
��2��ʵ���У����÷�̪��ָʾ���⣬������ѡ��___����ʵ���ָʾ��������ƿ�µ�һ�Ű�ֽ��������___��
��3������жϵζ��ﵽ���յ㣿___
��4������ʵ�����ݣ���������Һ��Ũ��Ϊ___mol��L-1��������λС������
��5�����в����п���ʹ�����ռ���Һ��Ũ����ֵƫ�͵���___��������ȷ�𰸱�ţ�
A.����b�У�δ��ϴ��ʽ�ζ���
B.����e�У���ʽ�ζ��ܼ��촦������δ�ϳ�����ȡҺ������촦��������ʧ��
C.����d�У�װ��Һʱ����ƿ�л�����������ˮ
D.����c�У��ζ��յ�ʱ������ʽ�ζ��ܼ��촦������һ����Һ
E.��ʽ�ζ��ܶ���ʱ���ζ�ǰ���Ӷ������ζ�����ʱ���Ӷ���