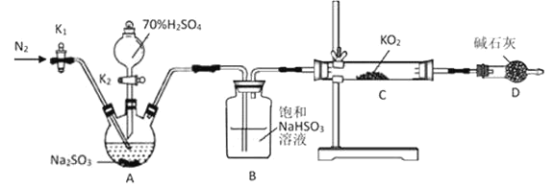
��Ŀ����
����Ŀ�������⻯�����Ϊ����������ʲ��ϣ�����Դ���������õ���������о���
��:��Mg(NH2)2 ��NaNH2 �� H3N��BH3 ��NaAlH4 ��Li3AlH6
(1)�����⻯�����¼��ȿ��ֽ�ų������������ϵ�λ���������������⻯���䴢��������͵���__________(����)��
(2)��Mg(NH2)2��NaNH2�о�����NH2����NH2���Ŀռ乹��Ϊ_________������ԭ�ӵ��ӻ���ʽΪ____________��
(3)H3N��BH3��ˮ��Ӧ����һ���κ�H2�Ļ�ѧ����ʽ��_____________________��д����̬Bԭ�ӵļ۵��ӹ������ʽ��__________________________��
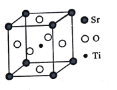
(4)Li3AlH6����ľ�������Ϊa��b��801.7 pm��c��945.5 pm����������90��������120�����ṹ��ͼ��ʾ��
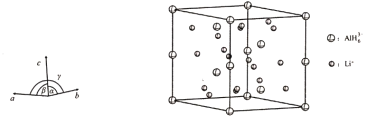
����֪AlH63���ķ�������Ϊ(0��0��0)��(0��0��![]() )��(
)��(![]() ��
��![]() ��
��![]() )��(
)��(![]() ��
��![]() ��
��![]() )��(
)��(![]() ��
��![]() ��
��![]() )��(
)��(![]() ��
��![]() ��
��![]() )��������Li���ĸ���Ϊ____________��
)��������Li���ĸ���Ϊ____________��
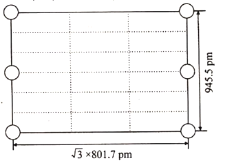
����ͼ������Li3AlH6������ij�����棬������10��AlH63��������6���Ѿ�����(ͼ�е���)������ͼ��������ʣ���AlH63������____________��
�۴˾�����ܶ�Ϊ____g��cm��3(�г�����ʽ����֪�����ӵ�����ԼΪ6.02��1023mol��1)��
���𰸡��� V�� sp3�ӻ� H3N��BH3+2H2O = NH4BO2 +3H2 ![]() 18
18  ��
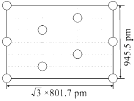
��
![]()
��������
(1)�����ϵ�λ���������⻯���䴢��������Ϊ�����⻯������Ԫ�ص�������������Mg(NH2)2��H��Ϊ![]() ����NaNH2��H��Ϊ
����NaNH2��H��Ϊ![]() ���� H3N��BH3��H��Ϊ
���� H3N��BH3��H��Ϊ![]() ����NaAlH4��H��Ϊ
����NaAlH4��H��Ϊ![]() ����Li3AlH6��H��Ϊ
����Li3AlH6��H��Ϊ![]() �����Դ���������͵�������
�����Դ���������͵�������
(2) NH2���ļ۲������Ϊ![]() ������VSEPR���ۣ�NH2�������۹���Ϊ�������壬�����Թ¶Ե��ӣ�����NH2���Ŀռ乹��ΪV�Σ���۲���Ӷ���Ϊ4����������ԭ��N���ӻ���ʽΪsp3�ӻ���
������VSEPR���ۣ�NH2�������۹���Ϊ�������壬�����Թ¶Ե��ӣ�����NH2���Ŀռ乹��ΪV�Σ���۲���Ӷ���Ϊ4����������ԭ��N���ӻ���ʽΪsp3�ӻ���
(3)���ݵ縺����ֵ��H3N��BH3�е�ԭ���ϵ���ԭ�Ӵ�����ɣ���ԭ���ϵ���ԭ�Ӵ�����ɣ�����H3N��BH3��ˮ��Ӧʱ����BH3�е���ԭ����ˮ�������з�Ӧ������������BH3ת��ΪB(OH)4-����ɼ�дΪBO2-�������Է�Ӧ�Ļ�ѧ����ʽΪ��H3N��BH3+2H2O = NH4BO2 +3H2��
���ǵ�5��Ԫ�أ���Ԫ�����ڱ���λ�ڵڶ����ڵ���A�塢p�������̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p1���۵����Ų�ʽΪ2s22p1���۵��ӹ������ʽΪ![]() ��
��
(4) ������AlH63���ķ�����������ж����ھ����е�λ�ã�8���ڶ��㡢4���ڲ������ġ�4���ھ������ڣ��ɷ�̯���ɵ�һ�������к���6��AlH63��������һ��������������Li+�ĸ���Ϊ18��
���ɽ���ı߳�(![]() 801.7pm��945.5pm)��֪���ǹ��������泤��ĺ����,��Ŀ���������������һ������10��AlH63�� ���ֻ���4��λ�ڶ��㡢2��λ�����ĵ�AlH63����������Ҫ��������4��λ�ھ�.�����ڵ�AlH63�������AlH63���ķ������꣬���ɽ�ȱʧ��AlH63�������������õ���ͼ��
801.7pm��945.5pm)��֪���ǹ��������泤��ĺ����,��Ŀ���������������һ������10��AlH63�� ���ֻ���4��λ�ڶ��㡢2��λ�����ĵ�AlH63����������Ҫ��������4��λ�ھ�.�����ڵ�AlH63�������AlH63���ķ������꣬���ɽ�ȱʧ��AlH63�������������õ���ͼ�� ��
��
�����ݾ����ܶȹ�ʽ��![]() ��������M=54g/mol��Z=6��V=801.72
��������M=54g/mol��Z=6��V=801.72![]() 945.5
945.5![]() sin60
sin60![]() pm3�����Ըþ�����ܶ�Ϊ
pm3�����Ըþ�����ܶ�Ϊ![]() ��
��

 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�