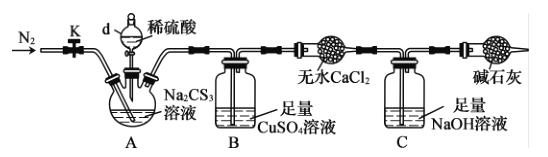
��Ŀ����
����Ŀ�����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000 mol��L��1��HCl����Һ�����к͵ζ����ü�����ָʾ��������ش��������⣺
(1)�ζ�ʱ��ʢװ����NaOH��Һ����������Ϊ_______________��
(2)ʢװ���������������Ϊ________________��
(3)�ζ����յ����ɫ�仯Ϊ_______________________________________________________��
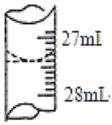
(4)����ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ0.50 mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ____________��
(5)������Щ������ʹ�ⶨ���ƫ��_________������ĸ����
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
���𰸡���ƿ ��ʽ�ζ��� ��ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ 26.90 mL AC
��������
(1)�ζ�������Һ�ڵζ����У�����Һʢ����ƿ�У�
(2)������Һ�������ʽ�ζ����У�
(3)���ݵζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���ұ��ְ���Ӳ���ɫ��
(4)���ݵζ��ܵĽṹ�뾫ȷ��Ϊ0.01mL��
(5)����![]() ��������������V��������Ӱ�죬�Դ��жϡ�
��������������V��������Ӱ�죬�Դ��жϡ�
(1)�ü�ʽ�ζ���ȡ�������NaOH��Һ����ƿ�У��ʴ�Ϊ����ƿ��
(2)ʢװ���������������Ϊ��ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ�
(3)����Һ���������ƣ���ƿ��ʢ�е�����������Һ�е�����ȣ���Һ����ɫ�ǻ�ɫ��������Һ��pH��С�����ε���Һ��pHС��4.4ʱ����Һ��ɫ�ɻ�ɫ��ɳ�ɫ���Ұ���Ӳ���ɫ���ε��������ʴ�Ϊ����Һ�ɻ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��
(4)��ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ0.50mL���ζ���Һ����ͼΪ27.40ml���ζ����е�Һ�����Ϊ27.40ml-0.50mL=26.90mL���ʴ�Ϊ��26.90mL��
(5)A����ƿ������ˮϴ�������ô���Һ��ϴ����ʹ��ƿ�����ʵ����ʵ����������V������ƫ����![]() ���������c�����⣩ƫ�ߣ���A��ȷ��
���������c�����⣩ƫ�ߣ���A��ȷ��
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ����V��������Ӱ�죬����![]() ���������c�����⣩���䣬��B����
���������c�����⣩���䣬��B����
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�������V������ƫ����![]() ���������c�����⣩ƫ�ߣ���C��ȷ��
���������c�����⣩ƫ�ߣ���C��ȷ��
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ����������V������ƫС������![]() ���������c�����⣩ƫ�ͣ���D����
���������c�����⣩ƫ�ͣ���D����
�ʴ�ΪAC��

 ���¿쳵����������ϵ�д�
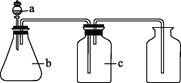
���¿쳵����������ϵ�д�����Ŀ����ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ���������(����)
��� | ���� | a | b | c |
|
A | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� | |
B | CO2 | ���� | ̼��� | ����NaHCO3��Һ | |
C | NO | ϡ���� | ͭм | H2O | |
D | NO2 | Ũ���� | ͭм | NaOH��Һ |
A. AB. BC. CD. D