��Ŀ����
Ϊ�ⶨij�л�������X�Ľṹ����������ʵ�飺
��1��ȡһ�����л���X����������ȫȼ�գ��������8.8g CO2��3.6g H2O�����ı�״���µ�����5.6L����X�к��� ��Ԫ�ء�
��2���������Dz��A����Է�������Ϊ44����X�ķ���ʽΪ ��
��3�����ݼۼ����ۣ���д��X���ܵĽṹ��ʽ�������Ǻ���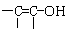 �ṹ�����ʣ�
��
�ṹ�����ʣ�
��
��4��X�ĺ˴Ź��������г���������ͬλ�õķ壬��X��������Ӧ�Ļ�ѧ����ʽ�� ��
��1��3 ��2��C2H4O
��3��CH3CHO ��
 (ÿ��1��)
(ÿ��1��)
��4��CH3CHO + H2 CH3CH2OH
CH3CH2OH
��������
�����������1��n��CO2��=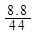 =0.2mol��n��H2O��=
=0.2mol��n��H2O��= =0.2mol��n��O2��=
=0.2mol��n��O2��= =0. 25mol
=0. 25mol
���������غ㶨�ɣ���̼�����⣬������Ԫ�أ����л����к�n��O��=0.2mol��2+0.2mol-0.5mol=0.1mol�����л�����N��C����N��H����N��O��=0.2mol��0.4mol��0.1mol=2��4��1��
��2���л����������Է�������Ϊ44��������ʵķ���ʽ��C2H4O��
���㣺�����л������ʽ�ͽṹʽ��ȷ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�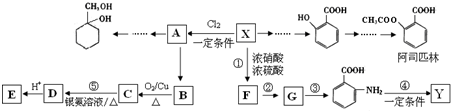
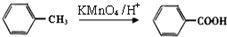
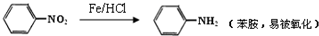
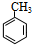
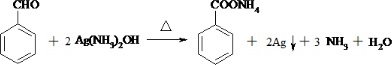

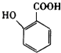 �ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯���ﹲ��
�ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯���ﹲ��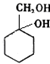 ������ͼ��
������ͼ��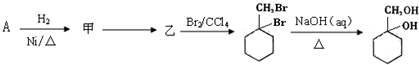

 12.0gijҺ̬�л�������A��ȫȼ�պ�����14.4gH2O��26.4g CO2������л�������A��������H2������ܶ���30����
12.0gijҺ̬�л�������A��ȫȼ�պ�����14.4gH2O��26.4g CO2������л�������A��������H2������ܶ���30����
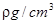 �����л���A����������ȫ��Ӧ����ͲҺ�����Ϊb mL����1 molA��������x mol��ԭ���ܸ������Ʒ�Ӧ����x�ļ���ʽΪ �����Բ�����
�����л���A����������ȫ��Ӧ����ͲҺ�����Ϊb mL����1 molA��������x mol��ԭ���ܸ������Ʒ�Ӧ����x�ļ���ʽΪ �����Բ�����
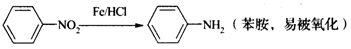
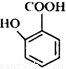 �ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯���ﹲ�� _________�֣�
�ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯���ﹲ�� _________�֣� ������ͼ��
������ͼ��