��Ŀ����
2��������������п��Ʒ������Fe2O3��FeO��CuO��Ϊԭ���Ʊ�����������п���仯ѧ����������ͼ1��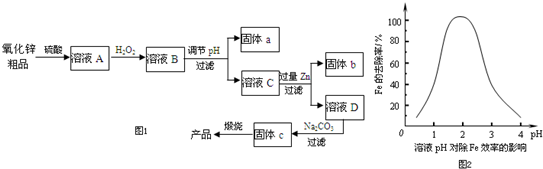
��Ҫ��ش��������⣺
��1����˫��ˮ��Ҫ������������Һ�е�Fe2+���÷�Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
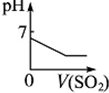
��2������pH��Ҫ��ʹ��Һ�е�Fe3+���ɳ���������ȥ����ҺpH�Գ�FeЧ��Ӱ����ͼ2��ʾ�����Fe3+ʱӦ������Һ��pHΪc������ţ���
a��3.5��4.0 b��2.5��3.5 c��1.5��2.5 d��0.5��1.5
��3������bΪCu��Zn���ѧʽ��������cΪ��ʽ̼��п�����չ���c�Ļ�ѧ����ʽΪZn2��OH��2CO3 $\frac{\underline{\;����\;}}{\;}$2ZnO+H2O+CO2����
��4����ҵ��Ҳ���Խ�����п��Ʒ���ü��ܵķ�����������пת��ΪNa2[Zn��OH��4]��Һ��Ȼ�������Һ��ȡп����ʯīΪ�缫���ʱ�������ĵ缫��ӦʽΪ[Zn��OH��4]2-+2e-=Zn+4OH-��������1molпʱ�����������������ڱ�״���µ����Ϊ11.2L����ֽ�ʵ�ء���ֽƬ�ڳ������ˮ������п��ɵĵ��Һ��ֽ��һ�߶�п��һ�߶ƶ������̣�����ܷ�ӦΪZn+2MnO2+H2O=ZnO+2MnO��OH�����õ�صĸ�����ӦʽΪZn+H2O-2e-=ZnO+2H+��
���� ����п��Ʒ������Fe2O3��FeO��CuO�����������ܽ�õ���Һ�к���FeSO4��Fe2��SO4��3��CuSO4��ZnSO4�������������������������Ϊ�����ӣ�������ҺPHʹ������ȫ�����������˵õ���Һ�м��������Zn����ͭ���ӻ�ԭΪCu�����ˣ�����bΪCu��ʣ���Zn����ҺD��Ҫ����ZnSO4������ҺD�м���̼��������Zn2��OH��2CO3�����ˣ�����Zn2��OH��2CO3 ����ZnO��
��1��H2O2��������Һ������Fe2+Ϊ�����ӣ�
��2������ͼ������ʹ�����ӳ�����
��3���������̷����ж�b�ijɷ֣���ʽ̼��п�����·ֽ�����ZnO��������̼��ˮ��
��4�����ʱ��[Zn��OH��4]2-�������õ�������Zn����������������ʧ�����������������ݵ����غ�����������������֪����ܷ�ӦΪZn+2MnO2+H2O=ZnO+2MnO��OH������Zn�ڸ���ʧ��������ZnO��
��� �⣺����п��Ʒ������Fe2O3��FeO��CuO�����������ܽ�õ���Һ�к���FeSO4��Fe2��SO4��3��CuSO4��ZnSO4�������������������������Ϊ�����ӣ�������ҺPHʹ������ȫ�����������˵õ���Һ�м��������Zn����ͭ���ӻ�ԭΪCu�����ˣ�����bΪCu��ʣ���Zn����ҺD��Ҫ����ZnSO4������ҺD�м���̼��������Zn2��OH��2CO3�����ˣ�����Zn2��OH��2CO3 ����ZnO��
��1��H2O2��������Һ������Fe2+Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��2������ͼ������ʹ�����ӳ�����ȫ��������ҺPH��2���ң���pHΪ1.5��2.5���ʴ�Ϊ��c��
��3�������̷�����֪b�ijɷ�ΪCu��Zn����ʽ̼��п�����·ֽ�����ZnO��������̼��ˮ���䷴Ӧ�ķ���ʽΪ��Zn2��OH��2CO3 $\frac{\underline{\;����\;}}{\;}$2ZnO+H2O+CO2����
�ʴ�Ϊ��Cu��Zn��Zn2��OH��2CO3 $\frac{\underline{\;����\;}}{\;}$2ZnO+H2O+CO2����
��4�����ʱ��[Zn��OH��4]2-�������õ�������Zn���������ĵ缫����ʽΪ��[Zn��OH��4]2-+2e-=Zn+4OH-����������������ʧ�������������������ĵ缫����ʽΪ��4OH--4e-�TH2O+O2�������Եõ���ϵʽ��Zn��2e-��$\frac{1}{2}$O2������1molпʱ�����ɵ�O2Ϊ0.5mol����11.2L����֪����ܷ�ӦΪZn+2MnO2+H2O=ZnO+2MnO��OH������Zn�ڸ���ʧ��������ZnO���为���ĵ缫����ʽΪ��Zn+H2O-2e-=ZnO+2H+��
�ʴ�Ϊ��[Zn��OH��4]2-+2e-=Zn+4OH-��11.2��Zn+H2O-2e-=ZnO+2H+��
���� ���⿼�������ʷ���ķ������ᴿ���̷����жϡ��Լ�ѡ��ͳ��ӵ���ҺpH�����жϡ�ԭ���ԭ���͵���ԭ����Ӧ�õȣ���Ŀ�漰��֪ʶ��϶࣬ע�����ԭ���ԭ���͵���ԭ���Լ��缫����ʽ����д��������Ŀ�Ѷ��еȣ�

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�| A�� | AlCl3 | B�� | Na2O | C�� | FeCl2 | D�� | SiO2 |
| A�� | 2CN-+Cl2����CN��2+2Cl- | B�� | ��CN��2+H2O��2H++CN-+CNO- | ||
| C�� | ��CN��2+2OH-��CN-+CNO-+H2O | D�� | ��SCN��2+2CN-��2SCN-+��CN��2 |
| A�� | 18O2��16O2�����ֲ�ͬ��ԭ�� | B�� | �״���CH3OH���������ӻ����� | ||
| C�� | N5��N2�ǵ�Ԫ�ص�����ͬλ�� | D�� | ��N5���N2�ǻ�ѧ�仯 |
| A�� | 4����ҺpH�Ĵ�С˳�٣��ܣ��ۣ��� | |
| B�� | �١��ڻ�Ϻ�pH��7��������Һ��c��NH4+����c��NH3•H2O�� | |
| C�� | �١����зֱ����25 mL 0.1 mol•L-1�������Һ��c��NH4+�����٣��� | |
| D�� | �ۡ����зֱ����12.5 mL 0.1mol•L-1 NaOH��Һ������Һ������������ͬ |
| A | B | C | D |
| ����������ͨ�� ��һ������ˮ�� | ����ˮ���뵽һ�� ���Ȼ�����Һ�� | ��ͭ�ۼ��뵽 һ����Ũ������ | �����ۼ��뵽һ �����Ȼ�����Һ�� |
 |  |  |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
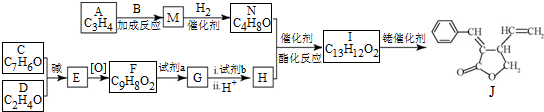

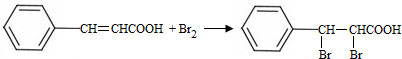 ���Լ�b��NaOH������Һ��
���Լ�b��NaOH������Һ��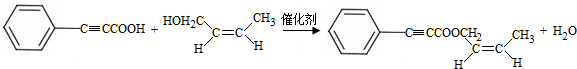 ��
��