��Ŀ����
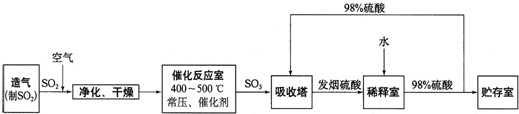
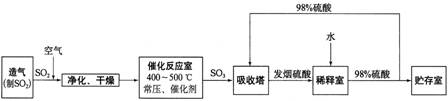
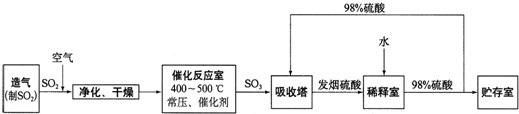
���Ṥҵ����������ʾ��
��1������Ӧ�ҷ�����Ӧ�Ļ�ѧ����ʽ�ǣ�______��
�÷�Ӧͨ����V2O5��������������������ǣ�V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2������д���ô�ѭ�������Ļ�ѧ����ʽ��______��
��2����������ͼ�ж�����˵����ȷ����______������ĸ����
a���������������SO2��ת����
b��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
c����98%����������SO3�����Ա����γ����������������
��3��ÿ160g SO3������H2O��l�����Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ����ʽ��______��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������˷�ֹSO2��Ⱦ�������õ�����⣬��ҪĿ���ǣ�______��
�⣺��1������Ӧ�ҷ����ķ�Ӧ�Ƕ�������Ĵ�������Ӧ������������Ӧ�Ļ�ѧ����ʽΪ��2SO2��g��+O2��g�� 2SO3��g�����ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ��������������Ӧ�Ļ�ѧ����ʽΪ��SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
2SO3��g�����ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ��������������Ӧ�Ļ�ѧ����ʽΪ��SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
�ʴ�Ϊ��2SO2��g��+O2��g�� 2SO3��g����SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
2SO3��g����SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
��2�����ݹ�ҵ�������̽�Ϸ�Ӧԭ��������
a������������������Ũ�ȣ������SO2��ת���ʣ���a��ȷ��
b���������Լӿ췴Ӧ���ʣ�ƽ�ⲻ������ʹ�ô����ܲ����SO2��ת���ʣ���b����
c����������������ˮ����98%����������SO3�����Ա����γ���������������ʣ���c��ȷ��
�ʴ�Ϊ��a��c��
��3��ÿ160g SO3������H2O��l�����Ϸų�260.6kJ��������80g����������ˮ��Ӧ����130.3KJ����Ӧ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������ն��������ֹ��Ⱦ�������õ���Ũ�ȵĶ��������˷�ֹSO2��Ⱦ�������õ�����⣬�ܵõ��ϸ�Ũ�ȵ�SO2��ʵ��ԭ��ѭ�������ã�
�ʴ�Ϊ���õ��ϸ�Ũ�ȵ�SO2��ԭ��ѭ�������ã�
��������1������Ӧ�ҷ����ķ�Ӧ�Ƕ�������Ĵ�������Ӧ�����������ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ������������
��2�����ݻ�ѧƽ��Ӱ�����غ�ƽ���ƶ�ԭ����ҵ�Ʊ�ԭ�������жϣ�
��3��ÿ160g SO3������H2O��l�����Ϸų�260.6kJ��������80g����������ˮ��Ӧ����130.3KJ�������Ȼ�ѧ����ʽ����д����д����
��4���ð�ˮ���գ�����Ũ���ᴦ����������������泥��ں����ᷴӦ����Ũ�ȸߵĶ�������ʵ��ԭ��ѭ�����ã�
���������⿼�������Ṥҵ�Ʊ�ԭ��Ӧ�ú�ע�����⣬��Ҫ�Ǵ����Ĵ�ԭ���������Ȼ�ѧ����ʽ��д����Ŀ�Ѷ��еȣ�
 2SO3��g�����ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ��������������Ӧ�Ļ�ѧ����ʽΪ��SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
2SO3��g�����ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ��������������Ӧ�Ļ�ѧ����ʽΪ��SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5���ʴ�Ϊ��2SO2��g��+O2��g��
 2SO3��g����SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
2SO3��g����SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5����2�����ݹ�ҵ�������̽�Ϸ�Ӧԭ��������
a������������������Ũ�ȣ������SO2��ת���ʣ���a��ȷ��
b���������Լӿ췴Ӧ���ʣ�ƽ�ⲻ������ʹ�ô����ܲ����SO2��ת���ʣ���b����
c����������������ˮ����98%����������SO3�����Ա����γ���������������ʣ���c��ȷ��
�ʴ�Ϊ��a��c��
��3��ÿ160g SO3������H2O��l�����Ϸų�260.6kJ��������80g����������ˮ��Ӧ����130.3KJ����Ӧ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������ն��������ֹ��Ⱦ�������õ���Ũ�ȵĶ��������˷�ֹSO2��Ⱦ�������õ�����⣬�ܵõ��ϸ�Ũ�ȵ�SO2��ʵ��ԭ��ѭ�������ã�
�ʴ�Ϊ���õ��ϸ�Ũ�ȵ�SO2��ԭ��ѭ�������ã�
��������1������Ӧ�ҷ����ķ�Ӧ�Ƕ�������Ĵ�������Ӧ�����������ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ������������
��2�����ݻ�ѧƽ��Ӱ�����غ�ƽ���ƶ�ԭ����ҵ�Ʊ�ԭ�������жϣ�
��3��ÿ160g SO3������H2O��l�����Ϸų�260.6kJ��������80g����������ˮ��Ӧ����130.3KJ�������Ȼ�ѧ����ʽ����д����д����
��4���ð�ˮ���գ�����Ũ���ᴦ����������������泥��ں����ᷴӦ����Ũ�ȸߵĶ�������ʵ��ԭ��ѭ�����ã�
���������⿼�������Ṥҵ�Ʊ�ԭ��Ӧ�ú�ע�����⣬��Ҫ�Ǵ����Ĵ�ԭ���������Ȼ�ѧ����ʽ��д����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ