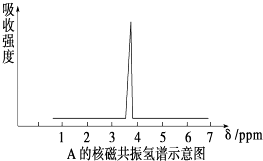
��Ŀ����
����Ŀ����ͼ�dz������ʼ��ת����ϵ�����мס��ҡ�����Ϊ�ǽ������ʣ�A��B��E�Ͷ���Ϊ�����B��EΪ�ܲ�������ЧӦ��������1mol E�к��� 10mol���ӣ��ҺͶ�Ϊ��ɫ���壬�����ǻ�ͼ��Ⱥ��ֹ����ɺ�ɫ��Ϊ��ɫ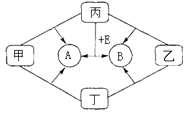
��1��д����ѧʽ��������������
��2������Ħ������Ϊ ��
��3��д������E��Ӧ����A��B�Ļ�ѧ����ʽ��
��4����ѧ�����ҺͶ���ͼ��Ⱥ��ռ�����״��������8.96L����ø����������������ܶ�Ϊ16����������ͨ�������ij���ʯ��ˮ�У��õ���ɫ������g��
���𰸡�
��1��H2��C��O2
��2��80g/mol
��3��CH4+2O2 ![]() ?CO2+2H2O
?CO2+2H2O
��4��10
���������⣺��������ЧӦ�������У�������̼�����顢�����ȣ������ĺ�ɫ�����У�̼���������̡����������������ۡ�����ͭ�ȣ����ҺͶ�Ϊ��ɫ��������Ϊ�ǽ������ʣ�������ΪC���ɱ��Ƿǽ������ʣ��Һͱ���Ӧ��������ЧӦ����B�����Ա���O2 �� B��CO2 �� B��EΪ�ܲ�������ЧӦ��������1mol E�к��� 10mol���ӣ���EΪCH4 �� �ٽ��ת����ϵ��֪��AΪˮ����ΪH2 �� ��ΪCuO����1��������������֪���ס��ҡ����ֱ�ΪH2��C��O2 �� ���Դ��ǣ�H2��C��O2�� ��2����ΪCuO����Է�������Ϊ80����Ħ������Ϊ80 g/mol�����Դ��ǣ�80 g/mol����3������E��Ӧ����A��B�Ļ�ѧ����ʽΪCH4+2O2 ![]() CO2+2H2O�����Դ��ǣ�CH4+2O2
CO2+2H2O�����Դ��ǣ�CH4+2O2 ![]() CO2+2H2O�� ��4����ø����������������ܶ�Ϊ16����MΪ16��2=32g/mol����״��������8.96L��ΪCO��CO2�Ļ���n=
CO2+2H2O�� ��4����ø����������������ܶ�Ϊ16����MΪ16��2=32g/mol����״��������8.96L��ΪCO��CO2�Ļ���n= ![]() =0.4mol����CO2�����ʵ���Ϊxmol����
=0.4mol����CO2�����ʵ���Ϊxmol���� ![]() =32�����x=0.1mol����Cԭ���غ��֪��n��CO2��=n��CaCO3��=0.1mol�����ɫ������Ϊ0.1mol��100g/mol=10g�����Դ��ǣ�10��
=32�����x=0.1mol����Cԭ���غ��֪��n��CO2��=n��CaCO3��=0.1mol�����ɫ������Ϊ0.1mol��100g/mol=10g�����Դ��ǣ�10��

 �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�����Ŀ������ʵ������������ȷ����(����)
ѡ�� | ʵ �� | �� �� |
A | ��Na2CO3��Һ��ͨ��������CO2���� | ���������� |
B | �ھƾ����ϼ������� | �����ۻ���ʧȥ�����ۻ��������������� |
C | ������ڿ����е�FeSO4��Һ�еμ�NaOH��Һ | ���̲���������ɫ���� |
D | ���ȷ��������е�С���� | �����ۻ��ɹ�����С��ȼ��ʱ����Ϊ��ɫ��ȼ�պ����ɵ���ɫ���� |
A. A B. B C. C D. D
����Ŀ���Ի�����Ϊԭ��������������������к�Fe2O3��SiO2��Al2O3��MgO�ȣ����������Ʊ����죨Fe2O3���Ĺ������£�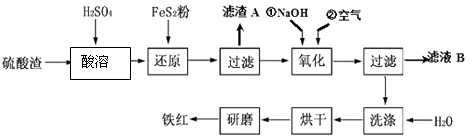
��1�����ܹ�����Fe2O3��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��������A����Ҫ�ɷݵĻ�ѧʽΪ ��
��2����ԭ�����м���FeS2��Ŀ���ǽ���Һ�е�Fe3+��ԭΪFe2+ �� ������������ΪH2SO4 �� ����ɸ÷�Ӧ�����ӷ���ʽ��FeS2+14Fe3++H2O�T15Fe2++SO ![]() + ��
+ ��
��3�����������У�O2��NaOH��Fe2+��Ӧ�����ӷ���ʽΪ ��
��4��Ϊ��ȷ�����������������������Ҫ������Һ��pH�ķ�Χ�����������ӳ�����pH���±�������ҺB���Ի��յ������У�д��ѧʽ�� ��
������ | Fe��OH��3 | Al��OH��3 | Fe��OH��2 | Mg��OH��2 |
��ʼ����pH | 2.7 | 3.8 | 7.6 | 9.4 |
��ȫ����pH | 3.2 | 5.2 | 9.7 | 12.4 |