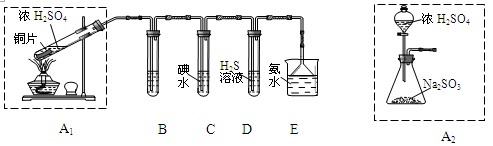
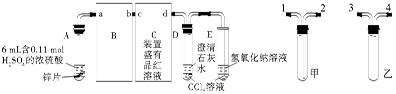��Ŀ����
�������ȣ�ClO2���ڳ�������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11��0�棬������ˮ����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶá�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2��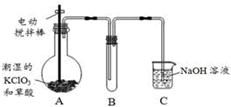
��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��
Bװ�ñ�����ڱ�ˮԡ�У���ԭ���� ��
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2���벹���NaClO2��Һ���Ƶ�NaClO2����IJ������裺�� ���� ����ϴ�ӣ��ܸ��
��4��ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10��00 mL��ϡ�ͳ�100��00 mL��������ȡV1mL�������뵽��ƿ�У�
����2����ϡ�������������pH��2��0������������KI���壬����Ƭ�̣�
����3���������ָʾ������cmol/LNa2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2mL������֪2 Na2S2O3+ I2��Na2S4O6+ 2NaI��
������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ����������У� ��
�ڵζ��������������������ƽ�вⶨ��ԭ���� ��
��д������2�з�����Ӧ�����ӷ���ʽ ��
��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����
�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ��� ��
���ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ��������ⶨ��� ��
���ƫ�ߡ���ƫ�͡����䡱 ��
��1��2KClO3+H2C2O4 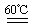 K2CO3+CO2��+2ClO2��+H2O��3�֣�
K2CO3+CO2��+2ClO2��+H2O��3�֣�
��2���¶ȼƣ�1�֣� ʹClO2������������ٻӷ���1�֣�
��3���������ᾧ��1�֣�д������Ũ�����ᾧ�����֣� �ڳ��ȹ��ˣ�2�֣�
��4����100ml ����ƿ����ͷ�ιܣ���1�֣�
�ڼ�����1�֣�
��2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O��2�֣�
��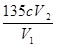 ��3�֣���ƫ�� ƫ�ͣ���1�֣�
��3�֣���ƫ�� ƫ�ͣ���1�֣�
���������������1�����������Ϣ֪��A�з�ӦΪ�Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ����K2CO3��ClO2��CO2��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��2KClO3+H2C2O4 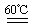 K2CO3+CO2��+2ClO2��+H2O����2��A���������¶ȿ���װ�ã�Ҫ�����¶ȱ���ʹ���¶ȼƲ����¶ȣ���Ӧ��60��ʱ���У�Ӧ��ˮԡ���ȣ����ƾ����⣬����Ҫ�IJ����������ձ����¶ȼƣ�Bװ��Ϊ�������ȵ��ռ�װ�ã��������ȣ�ClO2���ķе�Ϊ11��0�棬������ڱ�ˮԡ�У���ԭ����ʹClO2������������ٻӷ�����3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����NaClO2��Һ���Ƶ�NaClO2����IJ������裺�������ᾧ���ڳ��ȹ��ˣ���ϴ�ӣ��ܸ����4��������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ����������У�100 mL����ƿ����ͷ�ιܣ��ڵζ��������������������ƽ�вⶨ��ԭ���Ǽ������۲���2�з�����ӦΪ����������⻯�������������·�Ӧ���ɵ��ʵ⡢�Ȼ��غ�ˮ�����ӷ���ʽΪ2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O���ܸ��������Ӧ�ù�ϵʽ��ClO2����5Na2S2O3������������ݼ����ClO2��Һ��Ũ��Ϊ
K2CO3+CO2��+2ClO2��+H2O����2��A���������¶ȿ���װ�ã�Ҫ�����¶ȱ���ʹ���¶ȼƲ����¶ȣ���Ӧ��60��ʱ���У�Ӧ��ˮԡ���ȣ����ƾ����⣬����Ҫ�IJ����������ձ����¶ȼƣ�Bװ��Ϊ�������ȵ��ռ�װ�ã��������ȣ�ClO2���ķе�Ϊ11��0�棬������ڱ�ˮԡ�У���ԭ����ʹClO2������������ٻӷ�����3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����NaClO2��Һ���Ƶ�NaClO2����IJ������裺�������ᾧ���ڳ��ȹ��ˣ���ϴ�ӣ��ܸ����4��������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ����������У�100 mL����ƿ����ͷ�ιܣ��ڵζ��������������������ƽ�вⶨ��ԭ���Ǽ������۲���2�з�����ӦΪ����������⻯�������������·�Ӧ���ɵ��ʵ⡢�Ȼ��غ�ˮ�����ӷ���ʽΪ2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O���ܸ��������Ӧ�ù�ϵʽ��ClO2����5Na2S2O3������������ݼ����ClO2��Һ��Ũ��Ϊ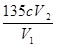 g / L�������ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ���������ı�Һ���ƫ����ⶨ���ƫ�ߣ����ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ�������������ı�Һ���ƫС����ⶨ���ƫ�͡�
g / L�������ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ���������ı�Һ���ƫ����ⶨ���ƫ�ߣ����ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ�������������ı�Һ���ƫС����ⶨ���ƫ�͡�
���㣺����ʵ�鷽������ơ����ۣ���ѧʵ�������������ѧ����ʽ����д����ϵʽ�����㡢�ζ���������������

��16�֣������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⣬ֱ���ŷź�SO2���������γ����꣬Σ��������
��1���û�ѧ����ʽ��ʾSO2�γ�����������ķ�Ӧ�� ��2��
��2����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L-1��Na2SO3��Һ����ҺpH���ϼ�С������ҺpHԼΪ6ʱ������SO2�����������½���Ӧ�������ռ���
�� ��ʱ��Һ��c(SO32�C)��Ũ����0.2 mol��L-1������Һ��c(HSO3�C)��_______mol?L-1��
�� ��pHԼΪ6�����ռ���ͨ��������O2���ɽ����е�NaHSO3ת��Ϊ�������ʣ���Ӧ�Ļ�ѧ����ʽ�� ��2��
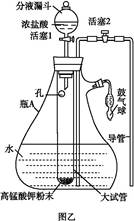
�� ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ��ģ��ʵ�����պ���������ʵ������ͼ��ʾ���� �����������SO2������Ч�ʡ�2��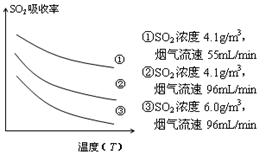
��3�������ֿ��ŵ�Na2SO3ҩƷ�Ѳ��ֱ������������û�ѧС��������֪Ũ�ȵ�����KMnO4��Һ��ȷ���京�������岽�����£�
����i����ȡ��Ʒ1.000 g��
����ii������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����iii����ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У���0.01000 mol��L��1 KMnO4����Һ�ζ����յ㡣
�����������������ظ�2�Ρ�
�� д������iii��������Ӧ�����ӷ���ʽ_________________________________��
�� ������0.01000 mol��L��1 KMnO4��Һʱ�����Ӷ��ݣ������ղ��ҩƷ��Na2SO3�ĺ���________(�ƫ����ƫС������Ӱ�족)��
�� ijͬѧ����������������еζ�ʵ��(�гֲ�����ȥ)�������������� (����ĸ)��

A B C D E
�� �ζ�������±���ʾ��
| �ζ����� | ������Һ �����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |
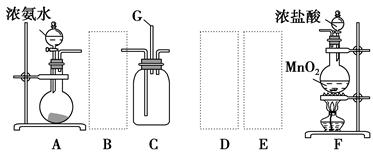
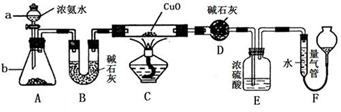
����������Ϣ��ҵ����Ҫ�Ļ������ϡ�ͨ���ý�̿�ڸ����»�ԭ���������Ƶôֹ�(������������������)���ֹ���������Ӧ�������Ȼ���(��Ӧ�¶�450��500 ��)�����Ȼ��辭�ᴿ����������ԭ�ɵøߴ��衣������ʵ�����Ʊ����Ȼ����װ��ʾ��ͼ��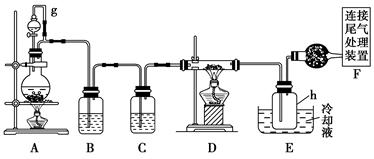
�����Ϣ���£�
a�����Ȼ�����ˮ����ˮ�⣻
b���������������ڸ����¾���������ֱ�ӷ�Ӧ������Ӧ���Ȼ��
c���й����ʵ������������±���
| ��� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| �е�/�� | 57.7 | 12.8 | �� | 315 | �� |
| �۵�/�� | ��70.0 | ��107.2 | �� | �� | �� |
| �����¶�/�� | �� | �� | 180 | 300 | 162 |
��ش��������⣺
(1)д��װ��A�з�����Ӧ�����ӷ���ʽ ____________________________��
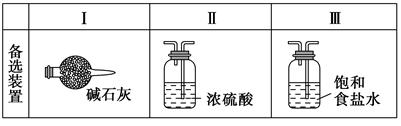
(2)װ��A��g�ܵ�������________��װ��C�е��Լ���________��װ��E�е�hƿ��Ҫ��ȴ��������________________________________________��
(3)װ��E��hƿ�ռ����Ĵֲ����ͨ������(���ƶ������)�õ��ߴ������Ȼ��裬�����IJ������У�����Ԫ������ܻ����е�����Ԫ����________(��дԪ�ط���)��
(4)Ϊ�˷�������������Ԫ�صĺ������Ƚ�������Ԥ������ʹ��Ԫ�ػ�ԭ��Fe2��������KMnO4����Һ�����������½���������ԭ�ζ�����Ӧ�����ӷ���ʽ�ǣ�5Fe2����MnO4����8H��=5Fe3����Mn2����4H2O
�ζ�ǰ�Ƿ�Ҫ�μ�ָʾ����________(��ǡ���)����˵������______________________��
��ijͬѧ��ȡ5.000 g�������Ԥ������������ƿ�����Ƴ�100 mL��Һ����ȡ25.00 mL������Һ����1.000��10��2 mol��L��1 KMnO4����Һ�ζ����ﵽ�ζ��յ�ʱ�����ı���Һ20.00 mL�������������Ԫ�ص�����������________��
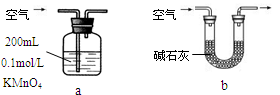
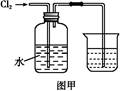
��ͼ��ijͬѧ�����ճ���Ʒע������Ƽ���ʵ��װ�á�����ע��10mL CH4��ͬ�¡�ͬѹ���ҹ���ע��50mL Cl2�����ҹ�����������У������ڼ��з�Ӧ��������չ�����һ��ʱ�䡣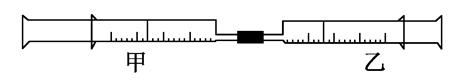
��1��������ijͬѧԤ���ʵ������
���������ձ�Ϊ��ɫ����ʵ������У��ܻ��������ƶ����ۼ��ڱ������飻�ܲ�����������ȷ����________��
��2�����з����Ļ�ѧ��Ӧ����Ϊ________��
��3����Ӧ����ʣ���������������Լ����յ���_________
____________��
| A��ˮ | B������������Һ |
| C����������Һ | D������ʳ��ˮ |