��Ŀ����
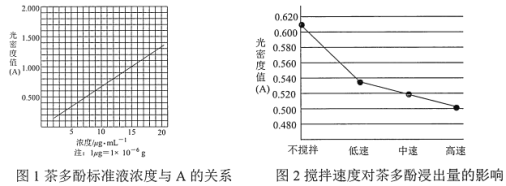
����Ŀ����Ҷ�еIJ�����һ����Ȼ�����������俹���������� VC �� 5��10 ��������������ˮ���Ҵ��������������������ȷ¡������Խ����У������ܽ� Fe3+��ԭΪ Fe2+��Fe2+�� K3Fe(CN)6���ɵ�����ɫ��λ������ KFe[Fe(CN)6]���ض�����������ճ̶ȣ��ù��ܶ�ֵ A ��ʾ���������һ��Ũ�ȷ�Χ�ڳ����ȡ�A ����ӱ�ҺŨ�ȵĹ�ϵ��ͼ 1 ��ʾ��
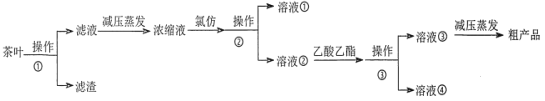
ijʵ��С���������ʵ�����̴Ӳ�Ҷ����ȡ���ӣ�
��ش��������⣺
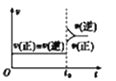
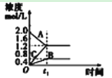
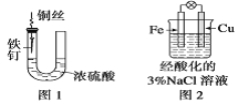
(1)��������ˮ��ȡ����ʱ��ʵ��С�鷢�ֽ����ٶȶԲ��ӽ�������Ӱ����ͼ 2 ��ʾ��ԭ����____�������Ҵ���ȡ���Ӳ������£���ȡ 10 g ��Ҷĩ������ֽ���ã�װ���ѹ��Һ©���У�Բ����ƿ�ڼӷ�ʯ�������Ҵ�����ͼ 3 ��װ�� ͨ����ˮ���������ȣ����Ҵ������ȷ��ں��Ƽ������¶��� 90�档Ϊʹ��ѹ©����Һ��߳���Ҷ��Լ 0.5 cm��������Լ 1 h�����еIJ���������________��
(2)��ѹ���������һ���������ŵ���________���ȷµ�������________��
(3)�����й�ʵ�������������ȷ����________��
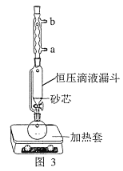
A��ͼ 3 ������ˮ����Ϊ a �� b ��
B����Һ©��ʹ��ǰ������Ƿ�©ˮ��ϴ������
C�������ٹ���ʱ�����ò������ʵ������Լӿ�����ٶ�
D����ȡ�����У�����ҡ����������Һ©��������Ȧ��������Һ
(4)��ȡ 1.25 g �ֲ�Ʒ��������ˮ�ܽⲢ������ 1000 mL����ȡ����Һ 1.00 mL���ӹ��� Fe3+�� K3Fe(CN)6 ������Һ��������ˮ������ 100 mL �����Һ���ܶ�ֵ A=0.800�����Ʒ�Ĵ�����_____ ��������������ʾ����
���𰸡������ױ����������������ٶ�Խ�죬���������ٶ�Խ�죬������Խ�� �رջ�������©����Һ��߳���Ҷ��Լ0.5cmʱ�����ڻ���ʹ�Ҵ������ٶ���©����Һ�ٶ�һ�� ���������¶ȷ�ֹ����������ֽ� ��ȡ������ӣ� CD 96%
��������
��Ҷ��ˮ����ʱ�����ӡ������ᡢ�����ܽ���ˮ�У����˺�ȥ���������õ�����Һ��ѹ�������ɽ��������¶ȣ���ֹ������Ӧ�ķ�������Ũ��Һ�м����ȷ¡���Һ���ɵú��ȷµ��л���Һ�٣�ˮ��Һ���к��в��ӣ�������������ȡ����Һ���ɵò��ӵ�����������Һ��Ȼ���ѹ���������ɻ�ò��Ӵֲ�Ʒ��
(1)��������ˮ��ȡ����ʱ����ͼ2�п��Կ�������������Խ�죬���ܶ�ֵ(A)ԽС��������Ũ��ԽС����Ϊ�����ױ���������������Խ�죬������ĽӴ�Խ�࣬�ɴ˵ó�ԭ���ǣ������ױ����������������ٶ�Խ�죬���������ٶ�Խ�죬������Խ�͡�Ϊʹ��ѹ©����Һ��߳���Ҷ��Լ 0.5 cm��������Լ 1 h�����еIJ��������ǹرջ�������©����Һ��߳���Ҷ��Լ0.5cmʱ�����ڻ���ʹ�Ҵ������ٶ���©����Һ�ٶ�һ�¡���Ϊ�������ױ����������������ٶ�Խ�죬���������ٶ�Խ�죬������Խ�ͣ��رջ�������©����Һ��߳���Ҷ��Լ0.5cmʱ�����ڻ���ʹ�Ҵ������ٶ���©����Һ�ٶ�һ�£�
(2)��ѹ����������������¶ȵͣ�����ʡ��Դ�⣬����ʹ�������¶Ƚ��ͣ����ŵ��ǽ��������¶ȷ�ֹ����������ֽ⣻��Ϊ����������������ȡ���ӣ����ȷ���ȡ��Ӧ�����ʣ��ɴ˵ó��ȷµ���������ȡ������ӣ�����Ϊ�����������¶ȷ�ֹ����������ֽ⣻��ȡ������ӣ���
(3)A��ͼ 3 ������ˮ����Ӧ�½��ϳ�����a��b����A��ȷ��
B����Һ©��ʹ��ǰ��Ϊ��©Һ��Ӧ��©��Ȼ��ϴ�ӱ��ã�B��ȷ��
C�������ٹ���ʱ�������ò��������裬����������ֽ��C����ȷ��
D����ȡ�����У���Һ©��Ӧ���÷ֲ���ٽ��з�Һ��D����ȷ��
��ѡCD����Ϊ��CD��
(4) ��Һ���ܶ�ֵ A=0.800���ӱ��пɲ�ò��ӵ�Ũ��Ϊ1.2��10-5g/mL���ɴ˿ɵó�ԭ1000 mL��Һ���������ӵ�����Ϊ1.2��10-5g/mL��100mL��![]() =1.2g�����Ʒ�Ĵ�����
=1.2g�����Ʒ�Ĵ�����![]() =96%����Ϊ��96%��
=96%������96%��

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
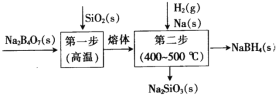
��Ԫ������ĩ��ϵ�д�����Ŀ������ԭ��ˮ���£�N2H4��H2O����һ��ǿ��ԭ�Եļ���Һ�塣
��ʵ��������ͼװ���Ʊ�ˮ���£�N2H4��H2O����
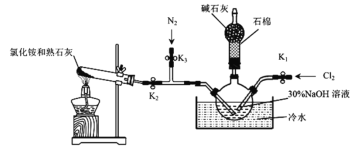
ʵ�鲽�裺�ر�K2��K3����K1���Ʊ� NaClO���ر�K1��K2����K3��ͨ��N2һ��ʱ�䣻�ر�K3����K2����ȼ�ƾ��ơ��ش��������⣺
��1��ʢ�ż�ʯ�ҵ�����������Ϊ___________________��
��2������30%NaOH��Һʱ�����貣���������ձ�������������ͷ�ιܺ�_________��
��3������2��ͨ��N2һ��ʱ���ԭ����____________________________________��
��4��ˮ���з�����ˮ��Ŀ����_____________________________________________��
��5��NH3�� NaClO��Ӧ����N2H4��H2O�Ļ�ѧ����ʽΪ_______________________��
����֪��N2H4��H2O+2I2=N2��+4HI+H2O���ⶨˮ���´ֲ�Ʒ�Ĵ��Ȳ������£�
a����ȡN2H4��H2O�ֲ�Ʒ(�������ʲ���I2��Ӧ)2.000g��
b����ˮ���250.00mL��Һ��
c���Ƴ�25.00mL������ƿ�У��μӼ��ε�����Һ��
d����0.3000mol��L��1�ĵ����Һ���еζ���
e���ظ������������Ρ����βⶨ�������±���
ʵ����� | 1 | 2 | 3 |
���ĵ�������/mL | 20.24 | 20.02 | 19.98 |
f�����ݴ�����
��6�������Һʢ����____________��������ʽ��������ʽ�����ζ����С��ڵζ�����װ�����ܵ�ǰһ����Ӧ���еIJ���Ϊ_________���ﵽ�յ��������__________��
��7�����ĵĵ����ƽ�����Ϊ______mL���ֲ�Ʒ��ˮ���µ���������Ϊ______��
��8���ж����в����Բⶨ�����Ӱ�죨����ƫ��������ƫ����������Ӱ��������
���������Ƶ����Һʱ���ձ��е���Һ��������������ⶨ���___________��
�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ⶨ���___________��