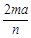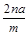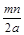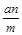��Ŀ����
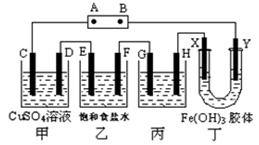
(10��)�ڳ��³�ѹ�£����ö��Բ������缫���ֱ���(ͬŨ�ȡ�ͬ�����ͨ����ͬ����)������Һ��
ͨ��һ��ʱ���жϵ�Դ������д���пհס�
(1)��A��һ���缫������������0��128 g����B��һ���缫���������� �����ӵ������� .
(2) ��һ������O2��ͬʱ��һ������H2(��д��ţ���ͬ)��
(3) ������Һ�е�C(OH��)����
(4)������������Һ��pH��С��
(5) ������Һ��Ũ�������[H+]���䣮
| A��CuSO4 | B��AgNO3 | C��KOH | D��H2SO4��E��NaCl F��Na2SO4 |
(1)��A��һ���缫������������0��128 g����B��һ���缫���������� �����ӵ������� .
(2) ��һ������O2��ͬʱ��һ������H2(��д��ţ���ͬ)��
(3) ������Һ�е�C(OH��)����
(4)������������Һ��pH��С��
(5) ������Һ��Ũ�������[H+]���䣮
(1)0.432g Ag (2)CDF (3)CE (4)ABD ��5��F
��1��A��һ���缫������������0��128 g����ͭ����0.128g��ת�Ƶ�����0.128g��64g/mol��2��0.004mol��B�����������������ݵ����غ��֪������0.004mol��������0.004mol��108g/mol��0.432g��
��2��һ������O2��ͬʱ��һ������H2��˵��������ˮ������������KOH��H2SO4��Na2SO4������ѡCDF��
��3������A��B��������ǿ��CDF��Ũ�ȶ�����C�м�����ǿ��D��������ǿ��F��pH���䡣����Ȼ��������������ƣ����Լ�����ǿ������3������4������5���Ĵ𰸷ֱ���(3)CE��(4)ABD����5��F��
��2��һ������O2��ͬʱ��һ������H2��˵��������ˮ������������KOH��H2SO4��Na2SO4������ѡCDF��
��3������A��B��������ǿ��CDF��Ũ�ȶ�����C�м�����ǿ��D��������ǿ��F��pH���䡣����Ȼ��������������ƣ����Լ�����ǿ������3������4������5���Ĵ𰸷ֱ���(3)CE��(4)ABD����5��F��

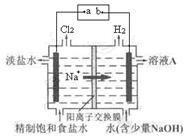
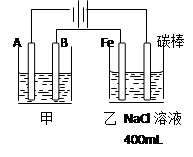
��ϰ��ϵ�д�
�����Ŀ