��Ŀ����
Ϊ�ⶨij��þ�Ͻ���þ������������ijС��ƻ�����þ�Ͻ�������ϡ������Һ��Ӧ���ⶨ����������������д���пհס�
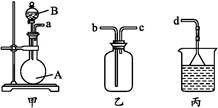
��1��ͬѧ��ѡ�ü�װ�ý���ʵ�飺ʵ�鿪ʼʱ���ȴ�Һ©���ϿڵIJ���������������������һ�����ϡ����Ͳ���˳��������ƿ�������������ԭ����________________��
��2��ͬѧ�Ǿ�������Ϊ��װ�����������������ϴ����ֱ���__________��__________��
��3���������������ʵ��װ���ҡ����е���a��������__________����ʵ��ǰ��ζ�����Һ������ֱ�ΪV1 mL��V2 mL����������������Ϊ______mL��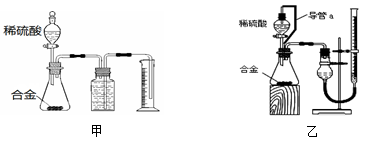
��1����1�֣�п��ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ���
��2����2�֣�ϡ���������ƿ�У�Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС��
��3����3�֣�ʹ��Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©����������������������ڼ���ϡ������������������ V1��V2
���������������1������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽�����������ѹǿԭ����ˮ�ų�����Ͳ��ˮ��������������������������Ͳ�ڵ���Ӧ������Ͳ�ײ���
��2��ϡ���������ƿ�У�Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС��
��3�����ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������ �ζ��ܵ���ֵ��̶����Ϸ������ε����֮��Ϊ�ⶨ�������������ע��Ӧ���ָ������ζ�����Һ��ȸߣ������Դ�V1��V2��
���㣺��������ļ���Ͳⶨ��

��һ��ʵ������Na2CO3��10H2O����500ml 0.10mol��L-1��Na2CO3��Һ����ղ���ش��������⣺
��1������ʵ��Ҫ�������
| Ӧ��ȡNa2CO3��10H2O������/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ�������������� |
| | | |
��2������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ����ĸֻ����һ��) ��
A��������ˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ�У���
B����������ƽȷ��������Na2CO3��10H2O�������������ձ��У��ټ�������ˮ���ò���������������ʹ���ܽ�(��Ҫʱ�ɼ���)��
C��������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3�������������������������ҺŨ�Ƚ��к�Ӱ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)?��û�н���A���� ����������ˮʱ���������˿̶� ��������ʱ���ӿ̶��� ��
��������Ȫʵ����һ�ֳ�������Ȼ���������ԭ���Ǵ���ѹǿ��Ը�����ͼ���ش��������⣺

��1����ͼA����ƿ�г����������壬��ͷ�ιܼ��ձ��зֱ�ʢ��Һ�塣��������в������γ���Ȫ���ǣ� ����
A��HCl��H��O B��Cl2��NaOH��Һ
C��HCl������ D��CO����NaOH��Һ
��2����ͼB����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ����� Ȫ���ǣ� ��
A��Cu��ϡ���� B��NaHCO����NaOH��Һ
C��CaCO����ϡ���� D��NH4HCO����ϡ����
��3����ͼB����ƿ���һˮ�ۣ���ƿ�м���ƾ���ˮ���м�����ˮ���ټ����������������ʣ����Ҳ��������Ȫ��ˮ���м�������ʲ������ǣ� ����
A��Ũ���� B����ʯ�� C������� D���ռ�
��4���Ƚ�ͼA��ͼB����װ�ã��Բ�����Ȫ��ԭ����������ͼA��_______�ϲ���ƿ��ѹǿ�������г�����������Ȫ����ɽ������ԭ��������________����ͼA��ͼB��װ�õ�ԭ�����ơ�
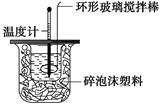
��10�֣�ijʵ��С����0��50 mol��L��1 NaOH��Һ��0��50 mol��L��1������Һ�����к��ȵIJⶨ��
������0��50 mol��L��1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����________g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(����ĸ)��__________��
| ���� | ������ƽ(������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ(�к���Ϊ57��3 kJ��mol��1)��_________________________
________________________________________________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
 �¶� �¶�ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26��2 | 26��0 | 26��1 | 30��1 | |
| 2 | 27��0 | 27��4 | 27��2 | 31��2 | |
| 3 | 25��9 | 25��9 | 25��9 | 29��8 | |
| 4 | 26��4 | 26��2 | 26��3 | 30��4 | |
������ʵ����ֵ�����57��3 kJ��mol��1��ƫ�����ƫ���ԭ�������(����ĸ)____________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ���ⶨ�����Ũ����ʵ�����н���ʵ�顣�������գ�
��1������100 mL 0.10 mol/L NaOH����Һ��
����Ҫ�������裺������������ܽ��(��ȴ��)ת�ơ�ϴ��(����ϴ��Һ��������ƿ)�� �������ƺõ���Һ�����Լ�ƿ�У����ϱ�ǩ��
�ڳ��� g�������ƹ������������У���ƽ(�����롢����)�� �� ��
��2��ȡ20.00 mL����������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�ҺNaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
| ʵ���� | NaOH��Һ��Ũ��(mol/L) | �ζ����ʱ�� NaOH��Һ��������(mL) | ����������Һ�����(mL) |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�ٵζ��ﵽ�յ�ı�־�� ��
�ڸ����������ݣ��ɼ�����������Ũ��Լ (������λ��Ч����)��
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е� ��ѡ��ס��ҡ���֮һ����Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
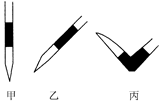
�� ������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ��У� ��
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D���ζ�������,��������Һ������ƿ�⡣
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
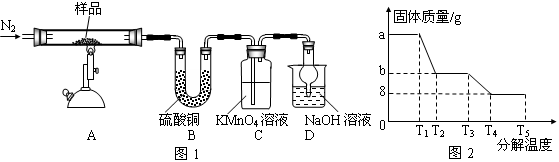
��������Ҫ�ɷ�ΪFeS2�����ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧѧϰС���ij������ʯ��������ʵ��̽����
[ʵ��һ]�ⶨ��Ԫ�صĺ�����
��m1 g�û�������Ʒ��������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2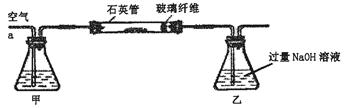
��Ӧ��������ƿ�е���Һ�������´�����
|

�������ۣ�
��1��I�У���ƿ����ʢ�Լ���____��Һ����ƿ�ڷ�����Ӧ�����ӷ���ʽΪ____��
��2��II�У���ƿ����H2O2��Һʱ��Ӧ�����ӷ���ʽΪ_____________________��
��3���û���������Ԫ�ص���������Ϊ_______________________��
��4��III�IJ�����У���Ҫ�õ����������ձ�������������ͷ�ι��⣬����____________________________________________��
��5��III�IJ�����У���ʾ�ζ��Ѵ��յ��������
��6����IJ���ܽ���������ƽ��ʵ�飬�������KMnO4��Һ����ֱ�Ϊ24.98mL��24.80mL��25.02mL��KMnO4����ԭΪMn2+���������������ݣ��ɼ�����û�������Ʒ��Ԫ�ص���������Ϊ ��