��Ŀ����
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ���ⶨ�����Ũ����ʵ�����н���ʵ�顣�������գ�
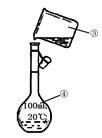
��1������100 mL 0.10 mol/L NaOH����Һ��
����Ҫ�������裺������������ܽ��(��ȴ��)ת�ơ�ϴ��(����ϴ��Һ��������ƿ)�� �������ƺõ���Һ�����Լ�ƿ�У����ϱ�ǩ��
�ڳ��� g�������ƹ������������У���ƽ(�����롢����)�� �� ��
��2��ȡ20.00 mL����������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�ҺNaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
| ʵ���� | NaOH��Һ��Ũ��(mol/L) | �ζ����ʱ�� NaOH��Һ��������(mL) | ����������Һ�����(mL) |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�ٵζ��ﵽ�յ�ı�־�� ��
�ڸ����������ݣ��ɼ�����������Ũ��Լ (������λ��Ч����)��
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е� ��ѡ��ס��ҡ���֮һ����Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
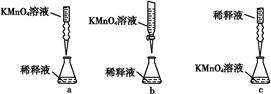
�� ������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ��У� ��
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D���ζ�������,��������Һ������ƿ�⡣
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
��1���ٶ��� ��0.4 �ձ�(�������ҩ��
��2�������һ��NaOH��Һ���룬��Һ����ɫǡ�ñ�ɷۺ�ɫ ��0.11 mol/L �۱� �� DE
������������������������1��������һ�����һ�����ʵ���Ũ�ȵ���Һ�IJ��������Ǽ�����������ܽ��(��ȴ��)ת�ơ�ϴ��(����ϴ��Һ��������ƿ)�����ݡ� ҡ�ȡ� װƿ ����ǩ����n(NaOH)=" 0.100" L ��0.10 mol/L=0.01mol.m(NaOH)=0.01mol��40g/mol=0.4g.���Ҫ����0.4gNaOH. ���������У���ƽ(�����롢����)\�ձ���ҩ�ס���2��������������֪Ũ�ȵļ�ζ�δ֪Ũ�ȵ��ᣬ����ָʾ��������Һ�У����ζ��ﵽ�յ�ʱ�ῴ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ����ȷ���ζ��ﵽ���յ㡣��V(NaOH)= (22.62��22.72��22.80)ml��3=22.71ml.��ΪHClǡ�÷�Ӧʱ���ʵ����ı�Ϊ1:1.����c(HCl)=" (" 0.10 mol/L��22.71ml)��20.00ml=" 0.11" mol/L. �� ��ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����еı�������Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ����A���ζ��յ����ʱ���Ӷ���������ƫС������Һ�������ƫС�������������Һ��Ũ�Ⱦ�ƫ�͡�����B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ�������������Һ�����ʵ�����ƫ�٣��ζ�ʱ���ĵı���Һ�����ƫС�������������Һ��Ũ�Ⱦ�ƫ�͡�����C����ƿˮϴ��δ�������Ӱ��ʵ��ⶨ���������D���ζ�������,��������Һ������ƿ�⡣Ϊ�˽�������Һ���еζ�����Ҫ��μӱ���Һ������Һ���ƫ���ɴ˼����������Һ��Ũ�Ⱦ�ƫ�ߡ���ȷ��E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ����ʼ����ƫС����������ƫС�������ĵı���Һ�������ƫ�࣬�ɴ˼����������Һ��Ũ�Ⱦ�ƫ�ߡ���ȷ��
���㣺��������к͵ζ��IJ������衢�ζ��յ���жϡ�Ũ�ȵļ��㼰ʵ����������֪ʶ��

ʵ���ҿ���K2Cr2O7������Ũ�����ϼ����Ʊ�������K2Cr2O7����ԭΪCr3+)��
(1)K2Cr2O7��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ_______������Ӧ��ת��3 mol e����������_______mol K2Cr2O7
(2)ϡ������K2Cr2O7�����ϼ���û���������ɡ�Ϊ̽��Ӱ���������ɵ����أ�ij��ѧ��ȤС���������ʵ�飺
���������
����1��Cl-Ũ�ȶԷ�Ӧ��Ӱ�죻
����2��H+Ũ�ȶԷ�Ӧ��Ӱ�죻
����3�� _____________________��
�����ʵ�鷽��������ʵ�顣д��ʵ�鲽�輰Ԥ������ͽ��ۡ�
��ѡ��ʵ���Լ���Ũ���ᡢϡ���ᡢŨ���ᡢNaOH��Һ��K2Cr2O7���塢NaCl���塢ʪ��ĵ���KI��ֽ
| ʵ�鲽�� | Ԥ������ͽ��� |
| ��:1����ʢ��K2Cr2O7�����A��B���Թ��зֱ����һ������ϡ���ᡣ | |
| ����2�� | |
| ����3�� | |
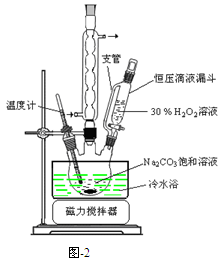
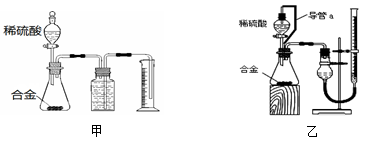
(14��)�������ҹ��ĺ�����ҵȡ���˾�ijɾͣ��ں��췢��ʱ����(N2H4)���������ﳣ��������ƽ�����
��Һ̬�������ȼ��ʱ����Һ̬N2O4��Ϸ���������ԭ��Ӧ����֪ÿ1 g�³�ַ�Ӧ��������̬ˮ�ų�����Ϊa KJ����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
����ʵ�����У���N2H4��H2O��NaOH����һ�������ռ�114��116 �����ּ�Ϊ��ˮ�¡�
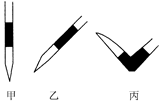
������������в���Ҫ�������� (�������ĸ)��
| A���ƾ��� | B����ֱ�������� | C����ƿ | D�������� |
�ڳ���������������⣬��ȱ�ٵIJ��������� ��
������ʹ��¯�ڱڵ�����(��Ҫ�ɷ�Fe2O3)��ɴ���������(Fe3O4)�㣬�ɼ�����¯��ʴ������Ӧ��������ת��Ϊ��������ÿ����1 mol Fe3O4����Ҫ�����µ�����Ϊ g��
�ȴ���������(Fe3O4)����ɿ�д��FeO��Fe2O3��ij��ѧʵ��С��ͨ��ʵ����̽��һ��ɫ��ĩ�Ƿ���Fe3O4��CuO���(������������ɫ����)��̽���������£�
������裺����1. ��ɫ��ĩ��CuO������2. ��ɫ��ĩ��Fe3O4��
����3. ��
���̽��ʵ�飺
ȡ������ĩ��������ϡ�����У���������Һ�еμ�KSCN�Լ���
��������1��������ʵ�������� ��
����������Һ��Ѫ��ɫ������� ������
��Ϊ��һ��̽����������������Һ�����������ۣ������� �����������3������
����һС��ͬѧ��������������CuO�������٣����ܼ������ۺ�ʵ���������ԡ�
�������ϣ�Cu2����������ˮ��Ӧ��������ɫ��Һ��Cu2����4NH3��H2O��Cu(NH3)42����4H2O��
��Ϊ̽���Ǽ���2���Ǽ���3��������ȡ������ĩ��ϡ�������ܽ���ټ���������ˮ��������2����������� ���������� ���������3������
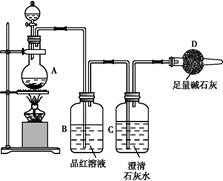
K3[Fe��C2O4��3]��3H2O[���������������ؾ���]������ˮ���������Ҵ�������Ϊ�л���Ӧ�Ĵ�����ʵ���ҿ�����мΪԭ���Ʊ�����ط�Ӧ�������¡���ش��������⣺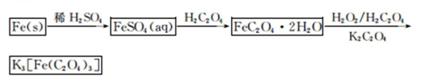
��1����м�г�����Ԫ�أ�������Ʊ�FeSO4ʱ������ж���H2S���壬�������������������Һ���ա���������װ����ȷ���� ������ţ���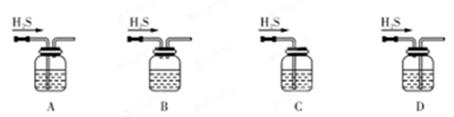
��2���ڵõ���FeSO4��Һ�������������H2 SO4�ữ��Ŀ���� ���õ�K3[Fe(C2O4)3]��Һ�����Ҵ���Ŀ���� ��
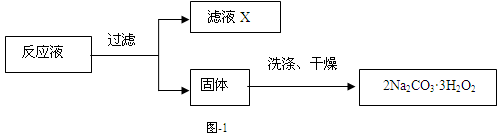
��3�������������ᾧˮ��ͨ�������������ⶨ����Ҫ�����У��ٳ����������ں������ѽᾧˮ������ȴ���ܳ��������ظ��ڡ��������أ����㡣
����ݵ�Ŀ���� ��
��4��C2O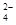 �ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
�ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
��д���ζ������з�����Ӧ�����ӷ���ʽ ��
�����еζ�������ʹ�ζ����ƫ�ߵ��� ������ţ���
| A���ζ���������ˮϴ�Ӻ�����װ���Һ |
| B����ƿ��װ����Һǰδ�ô���Һ��ϴ |
| C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ |
| D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ��� |
ʵ���ҳ����ü�ȩ��HCHO�����ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH4�� ��6HCHO =3H����6H2O��(CH2)6N4H�� �۵ζ�ʱ��1 mol (CH2)6N4H���� l mol H���൱��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
����I ��ȡ��Ʒ1��500 g��
����II ����Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����III ��ȡ25��00 mL��Ʒ��Һ��250 mL��ƿ�У�����10 mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
��1�����ݲ���III��գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����_______(�ƫ����ƫС������Ӱ�족)
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�____________��
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ����__________________________________________________��
��2���ζ�������±���ʾ��
| �ζ� ���� | ������Һ����� /mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25��00 | 1��02 | 21��03 |
| 2 | 25��00 | 2��00 | 21��99 |
| 3 | 25��00 | 0��20 | 20��20 |
��NaOH����Һ��Ũ��Ϊ0��1010 mol��L��1�������Ʒ�е�����������Ϊ___________��
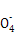 +8H+
+8H+ 5Fe3++Mn2++4H2O)��
5Fe3++Mn2++4H2O)��